
MED-EL
Published Apr 16, 2024
Replacing BAHA With BCI 602: Surgeons Agree on International Guidelines
The active, transcutaneous BONEBRIDGE BCI 602 implant offers clear advantages over percutaneous BAHA (bone-anchored hearing aid) implants. Time and again, BAHAs are explanted and replaced with BONEBRIDGE. Leading ENT surgeons have reached an international consensus and established guidelines for the explantation of a BAHA followed by implantation of BCI 602.
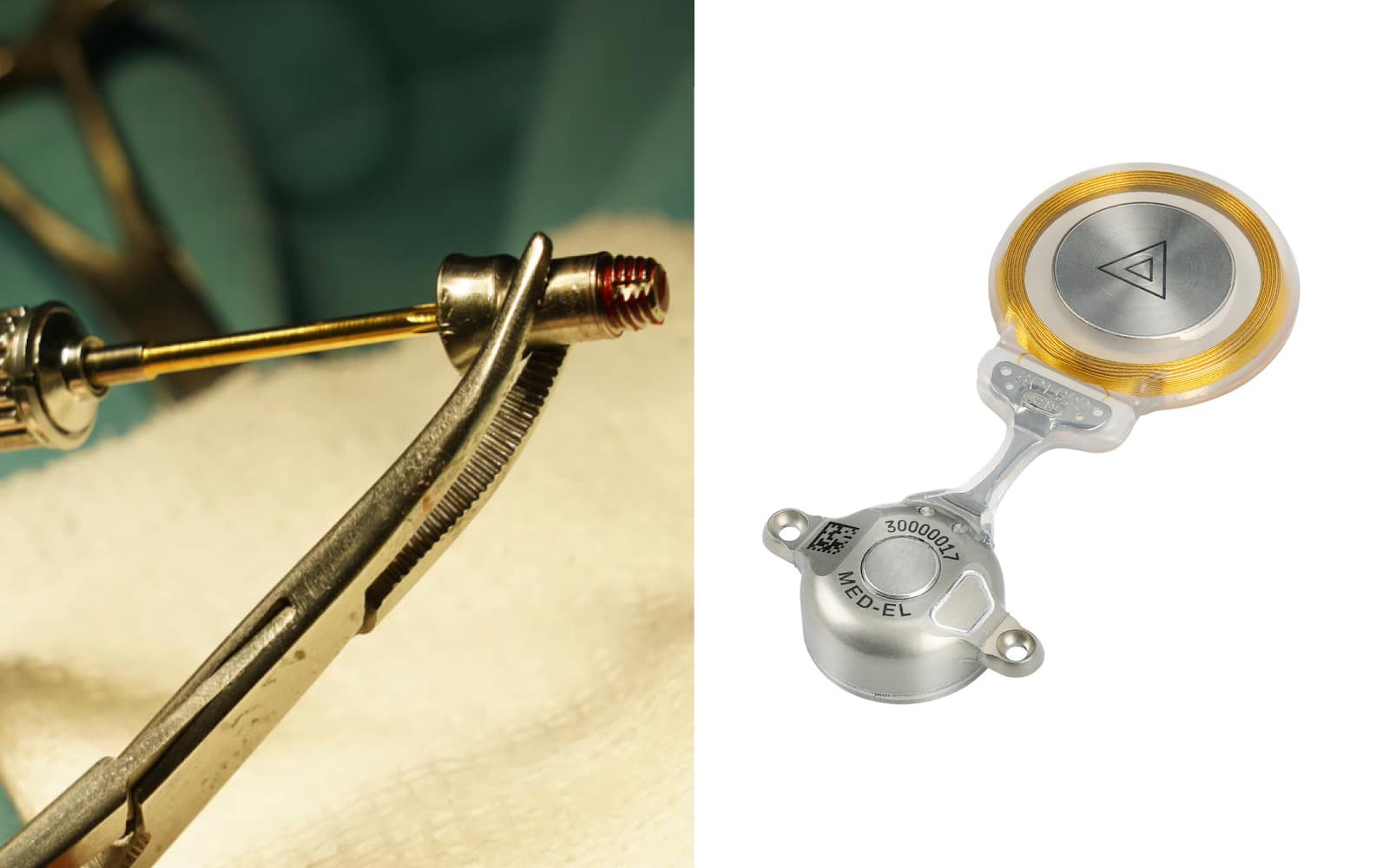
Fewer complications, no permanent wound, intact skin, maximum wearing comfort, and outstanding reliability—the advantages of the active, transcutaneous BONEBRIDGE bone conduction implant speak for themselves.
As opposed to percutaneous bone conduction implants that send vibrations to the bone skull through a screw that penetrates the skin, BONEBRIDGE combines the advantages of active stimulation with transcutaneous technology. The external audio processor sends signals through the intact skin to the implant, which produces vibrations that are transmitted directly to the bone. And because the microphones in the SAMBA 2 audio processor communicate with the BCI 602 via induction—but are physically separated—irritating feedback, which can be an issue for percutaneous systems, is prevented.
In short, BONEBRIDGE offers a comfortable, discreet, and powerful solution for people with conductive or mixed hearing loss or single-sided deafness:
- Active, transcutaneous technology
- Outstanding reliabilitySystematic review and meta-analysis of audiological and patient-reported outcomes with the BONEBRIDGE active bone conduction implant system. MED-EL 2022: https://go.medel.pro/OutcomesBB[1]
- Straightforward, safe surgeryMagele et al.: Active transcutaneous bone conduction hearing implants: systematic review and meta-analysis. PLoS One, 2019 Sep 16, 14 (9), e0221484.[2]Sprinzl et al.: A Surgical Experience and Early Audiological Outcomes with the new Active transcutaneous Bone Conduction Implant, Otol Neurotol, in press.[3]Yang et al: Audiological and subjective outcomes of 100 implanted transcutaneous bone conduction devices and preoperative bone conduction hearing aids in patients with bilateral microtia-atresia, Acta Otolaryngol 2020 Aug, 140 (8), 675-681.[4]
- Stable two-point fixation in the bone bed
- Excellent hearing resultsMagele et al.: Active transcutaneous bone conduction hearing implants: systematic review and meta-analysis. PLoS One, 2019 Sep 16, 14 (9), e0221484.[2]Sprinzl et al.: A Surgical Experience and Early Audiological Outcomes with the new Active transcutaneous Bone Conduction Implant, Otol Neurotol, in press.[3]Yang et al: Audiological and subjective outcomes of 100 implanted transcutaneous bone conduction devices and preoperative bone conduction hearing aids in patients with bilateral microtia-atresia, Acta Otolaryngol 2020 Aug, 140 (8), 675-681.[4]
- High user satisfaction and improved quality of lifeSprinzl et al.: A Surgical Experience and Early Audiological Outcomes with the new Active transcutaneous Bone Conduction Implant, Otol Neurotol, in press.[3]
International Guidelines for Conversions From BAHA to BCI 602
When it comes to hearing success and reliability, BONEBRIDGE is the clear winner over percutaneous systems.Systematic review and meta-analysis of audiological and patient-reported outcomes with the BONEBRIDGE active bone conduction implant system. MED-EL 2022: https://go.medel.pro/OutcomesBB[1] The increased risk of complications, extrusion and infection that comes with percutaneous bone conduction systems are to replace BAHAs with BONEBRIDGE implants.
Once the decision to explant BAHA and then implant BONEBRIDGE has been made, then the question about “how” arises. In the past, a variety of approaches and methods had been used, but just a few months ago, leading ENT surgeons from Europe and South America reached an international consensus about the recommended approach.
The participating surgeons were:
- Javier Gavilán, Hospital Universitario La Paz, Spain
- Burkhard Schwab, Helios Klinikum Hildesheim, Germany
- Mario Zernotti, Universidad Católica de Córdoba, Argentina
- Carlos Curet, National University of Cordoba, Argentina
- Diego Marcomini, Sanatorio Franchin, Argentina
- Andrea Albera, Città della Salute e della Scienza di Torino, Italy
- Andrea Canale, Città della Salute e della Scienza di Torino, Italy
- Arthur Castilh, Unicamp Hospital de Clinicas Campinas, Brazil

At a meeting in Denver, USA, the experts discuss what a standardized surgical procedure could look like based on numerous real cases involving various medical histories and complications.
The result is clear: despite the varying backgrounds of the participants (different clinical procedures, surgical schools, healthcare systems, etc.), they were able to come to a consensus about procedures and document them in the form of surgical guidelines: Conversion Type 1 and Conversion Type 2.
Preoperative Analysis and Classification of the Complications as Criteria
About 10% of people with a BAHA report major complications caused by the implant.Safety outcomes of bone conduction and middle ear implants: A systematic review, rev. 5.0. MED-EL 2020.[5] These complications are often infections or inflammation that cause damage to multiple layers of skin and lead to limited or discontinued use of the implant. The decision between Type 1 and Type 2 is made prior to BAHA explantation and is based on the reason for the conversion and the complications diagnosed preoperatively.
Examples of complications with BAHAs:
- infection
- inflammation
- hematoma (bruising)
- extrusion
- atrophic or necrotic tissue
- granulation tissue and growths
To make it easier to assess the severity of the complications, the guidelines rely on the Holgers scale: Grade 0 (no skin reaction), Grade 1 (redness with slight swelling), Grade 2 (redness, moistness, and moderate swelling), Grade 3 (redness, moistness, and moderate swelling with granulation tissue), Grade 4 (profound signs of infection resulting in removal of the implant).Holgers et al.: Soft tissue reactions around percutaneous implants: A clinical study of soft tissue reactions around skin-penetrating titanium implants for bone anchored hearing aids. Am J Otol. 1988, 9: 56- 59.[6]
An exact classification of the preoperative condition is necessary for every case because it determines all of the subsequent surgical steps. The following procedure is outlined in the guidelines: Levels 0-3 indicate Conversion Type 1, Level 4 requires Conversion Type 2.
Special Consideration in Surgical Planning
Both surgical procedures—Conversion Types 1 and 2—require exact preoperative planning, especially because the BAHA screw results in additional challenges for BCI 602 placement. It is crucial that the area in which the BCI will be placed is not damaged when the incision is made or during explantation of the BAHA. Preoperative visualization using OTOPLAN can offer assistance during planning BONEBRIDGE implantations. Now we will take a closer look at both conversion types.

Conversion Type 1
This procedure is used for grades 0, 1, 2, and 3 on the Holgers scale—when there are no profound signs of infection (Grade 4).
Phase 1: Explantation
Cut a Rhomboid (Limberg) incision as far away as possible from the position where you will later place the BCI. Then remove the BAHA screw. This is especially important when you suspect a bone infection. Tighten and then explant the screw using the appropriate counter torque wrench, forceps, and/or a cutting bur (2, 3, or 4 mm). To have as much control as possible and avoid damage to the dura, be sure to have a clear line of sight to the bone.
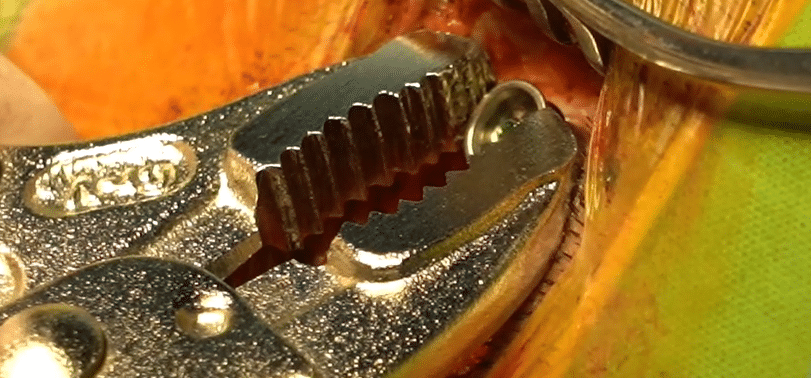
Phase 2: Implantation
After a 5-week healing period, BCI 602 can be implanted. The procedure is the same as a conventional BONEBRIDGE implantation—but potentially requires more flexibility when it comes to placement due to previous surgeries. And here an important advantage of the BCI 602 is clear: The implant has a flexible transition between the receiver coil and the transducer that .
Conversion Type 2
This procedure is used when there are clear signs of infection and/or the skin tissue is already dead (Holgers scale Grade 4).
Phase 1: Explantation
Explant BAHA as in Conversion 1—additionally, remove atrophic and poorly circulated skin tissue to achieve good skin vascularity. And this is where there is the most significant difference to Conversion Type 1: Due to the removal of skin, cut a bilobed flap, rotate 45°, and pull into place. It is important that the skin flap is as well-circulated as possible. Using a Z-shaped incision reduces skin tension so it can be sewn up afterward.
Phase 2: Implantation
The healing period is much longer for Type 2—due to the extent of tissue damage, it lasts at least 5 months. Once again, implantation of BCI 602 is done conventionally. But placement may proceed differently than usual due to the past BAHA explantation. And for better preoperative planning, OTOPLAN can help. The horizontal and vertical flexibility of the implant are also advantageous.
Receive More Information
If you would like to know more about these surgical procedures or the BONEBRIDGE bone conduction system, contact your local MED-EL team.[7]
For a case study related to this article, check out BONEBRIDGE Instead of BAHA: Case Study and Surgical Video.
Subscribe to the MED-EL Professionals Blog
Regularly receive information directly to your email inbox about new MED-EL products, interesting case studies, and updates related to ENT surgery, audiology, and hearing rehabilitation.
SubscribeReferences
-
[1]
Systematic review and meta-analysis of audiological and patient-reported outcomes with the BONEBRIDGE active bone conduction implant system. MED-EL 2022: https://go.medel.pro/OutcomesBB
-
[2]
Magele et al.: Active transcutaneous bone conduction hearing implants: systematic review and meta-analysis. PLoS One, 2019 Sep 16, 14 (9), e0221484.
-
[3]
Sprinzl et al.: A Surgical Experience and Early Audiological Outcomes with the new Active transcutaneous Bone Conduction Implant, Otol Neurotol, in press.
-
[4]
Yang et al: Audiological and subjective outcomes of 100 implanted transcutaneous bone conduction devices and preoperative bone conduction hearing aids in patients with bilateral microtia-atresia, Acta Otolaryngol 2020 Aug, 140 (8), 675-681.
-
[5]
Safety outcomes of bone conduction and middle ear implants: A systematic review, rev. 5.0. MED-EL 2020.
-
[6]
Holgers et al.: Soft tissue reactions around percutaneous implants: A clinical study of soft tissue reactions around skin-penetrating titanium implants for bone anchored hearing aids. Am J Otol. 1988, 9: 56- 59.
References

MED-EL
Was this article helpful?
Thanks for your feedback.
Sign up for newsletter below for more.
Thanks for your feedback.
Please leave your message below.
CTA Form Success Message
Send us a message
Field is required
John Doe
Field is required
name@mail.com
Field is required
What do you think?
The content on this website is for general informational purposes only and should not be taken as medical advice. Please contact your doctor or hearing specialist to learn what type of hearing solution is suitable for your specific needs. Not all products, features, or indications shown are approved in all countries.
Christine Kelly
June 06, 2024
Where in the USA is the BCI 602 performed?.
MED-EL
June 07, 2024
Hi Christine, thanks for your comment. We recommend getting in touch with your local MED-EL team via https://www.medel.com/en-us/contact-med-el for assistance. Alternatively, we recommend to search for a clinic in your area where BCI 602 is performed by adding BONEBRIDGE to the filter: https://www.medel.com/en-us/clinic-finder Kind regards, Gordana

MED-EL

MED-EL

.png)


Conversation
1 Comment