Nathan Schackow, Stefano Morettini
Published Oct 31, 2025
5 Ways to Maximize Residual Hearing Preservation With Precision Surgery
MED-EL has focused decades of research on residual hearing preservation. Here are the results, along with some of the secrets to our success.
Residual hearing preservation is essential for enhancing sound quality, speech perception in noise, and music perception following cochlear implantation.Schaefer, S., Sahwan, M., Metryka, A., Kluk, K., & Bruce, I. A. (2021). The benefits of preserving residual hearing following cochlear implantation: a systematic review. International Journal of Audiology, 60(8), 561–577. https://doi.org/10.1080/14992027.2020.1863484[24] At MED-EL, we have focused decades of deliberate research on residual hearing preservation, and here are the results, along with some of the secrets to our success.

“With our FLEX electrode design, MED-EL has engineered the only cochlear implants proven to preserve residual hearing in many recipients. We stimulate the entire cochlea with the right signal.”
Ingeborg Hochmair, Founder and CEO of MED-EL
Data from a systematic literature review demonstrate complete or partial residual hearing preservation in the majority of MED-EL recipients with FLEX electrodes.Heyning, P. H. V. de, Dazert, S., Gavilan, J., Lassaletta, L., Lorens, A., Rajan, G. P., Skarzynski, H., Skarzynski, P. H., Tavora-Vieira, D., Topsakal, V., Usami, S., Rompaey, V. V., Weiss, N. M., & Polak, M. (2022). Systematic Literature Review of Hearing Preservation Rates in Cochlear Implantation Associated With Medium- and Longer-Length Flexible Lateral Wall Electrode Arrays. Frontiers in Surgery, 9, 893839. https://doi.org/10.3389/fsurg.2022.893839[14]
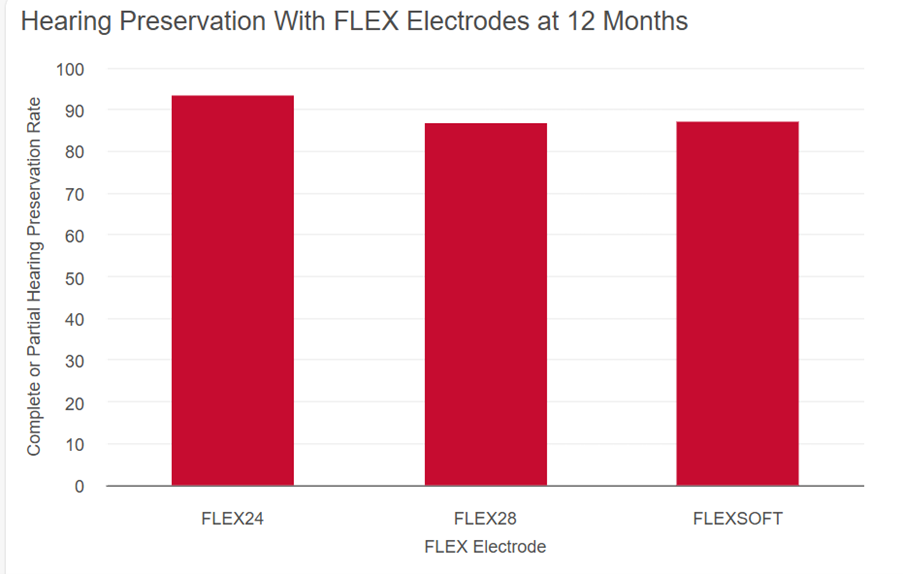
Adapted from Van de Heyning et al., 2022. For inclusion, insertion depths of at least 20 mm were reached with FLEX24 electrodes and over 28 mm with FLEXSOFT and FLEX28 electrodes in EAS patients.
The Only Cochlear Implants Granted FDA Approval for Residual Hearing Preservation
MED-EL is now the first and only cochlear implant manufacturer to be awarded FDA approval for residual hearing preservation.US Food & Drug Administration. (2024). Premarket approval, MED-EL Cochlear Implant System. P000025/S129. https://www.accessdata.fda.gov/cdrh_docs/pdf/P000025S129B.pdf[25] Based on anonymized registry results from a variety of surgeons and hospitals, the results show that MED-EL’s FLEX electrode design allows the majority of recipients to retain some or all functional residual hearing post-implantation.
More details on MED-EL’s FDA approval for residual hearing preservation, definitions, and what that means for cochlear implant candidacy in the United States can be found on the US MED-EL Professionals Blog here: FDA Expands MED-EL Criteria for Adults With Residual Hearing
While loss of residual hearing remains a risk with any cochlear implant surgery, thanks to MED-EL’s FLEX electrode design, it is no longer a given that the surgery will destroy all remaining hearing.
MED-EL Cochlear Implants: The Best Opportunity for Hearing Preservation
This FDA approval has important implications for how cochlear implantation is viewed by hearing professionals and cochlear implant candidates. A MED-EL CI with a FLEX electrode provides recipients the best opportunity to continue to benefit from their residual hearing and, in addition, gain the benefits of cochlear implantation. In other words, a MED-EL cochlear implant can supplement remaining natural hearing in the majority of cases.
With 24-31.5 mm electrode arrays, MED-EL’s unique FLEX electrode design shows some degree of hearing preservation 1-2 years after surgery. This was based on real-world data, not from a controlled study on hearing preservation. What this means is only beginning to sink in. Receiving a MED-EL cochlear implant is no longer an either-or decision in most cases—candidates can have the benefits of a CI and their residual hearing too—so clinicians should not delay seeking treatment or referring CI candidates with remaining hearing for a cochlear implant evaluation.
How Can Structural and Hearing Preservation Be Maximized?
Electrode design and surgical techniques are both required for hearing and structural preservation to be achieved.
1. Electrodes Designed for Hearing Preservation
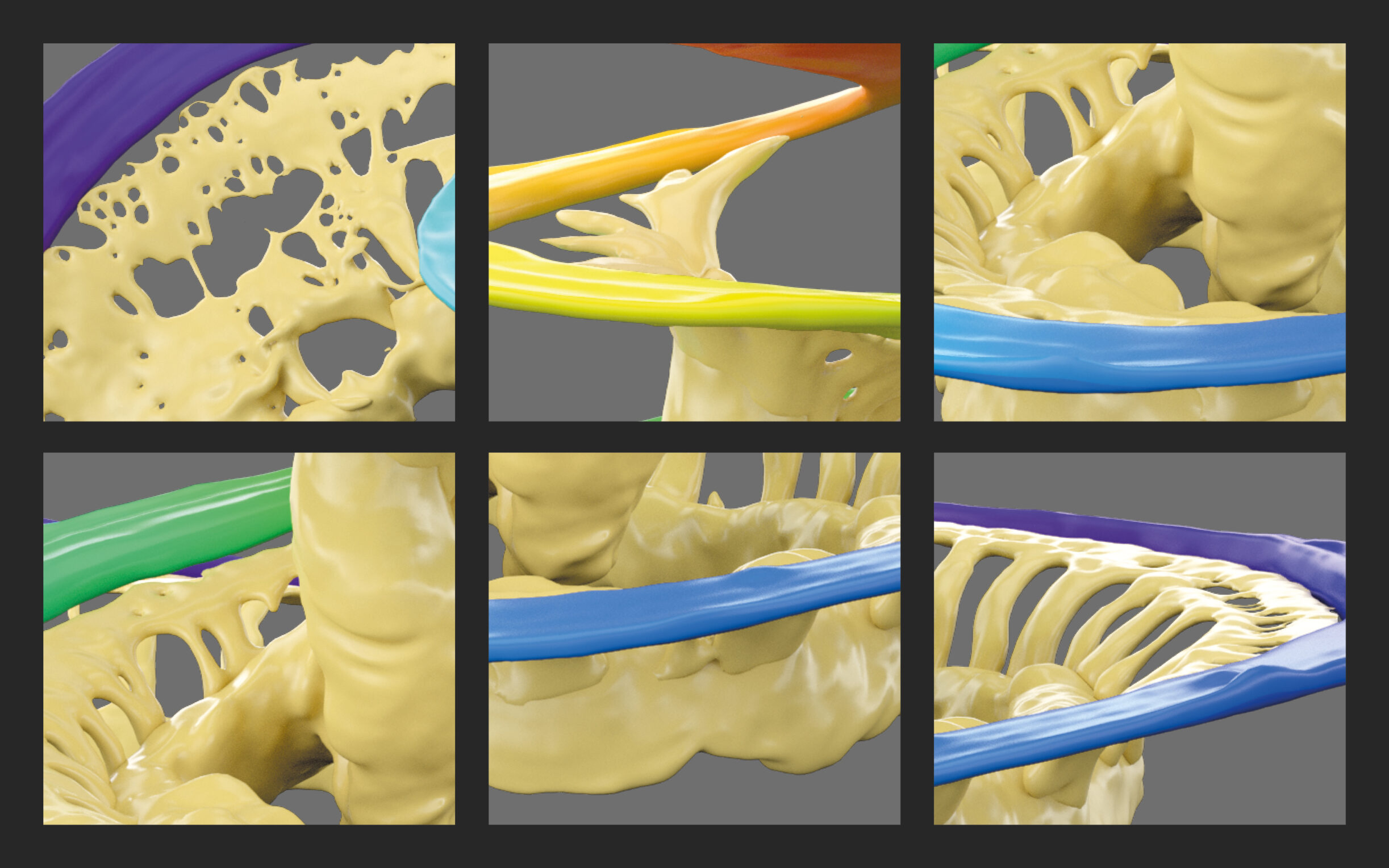
“Flexible and straight lateral wall electrode type is reported to be gentle to intra-cochlear structures and has the potential to electrically stimulate most of the neuronal elements, which are necessary in bringing full benefit of the CI device to recipients.”Dhanasingh, A., Nielsen, S. B., Beal, F., Schilp, S., Hessler, R., Jolly, C., & Hochmair, I. (2024). Cochlear implant electrode design for safe and effective treatment. Frontiers in Neurology, 15, 1348439. https://doi.org/10.3389/fneur.2024.1348439[11]
MED-EL’s FLEX lateral wall electrodes are uniquely soft and mechanically flexible to gently adapt to the unique shape of each cochlea while bringing contacts close to neural targets.

- Wave-shaped wires bend easily, which helps reduce rigidity and insertion forces in comparison to straight-wire designs.Dhanasingh, A., & Jolly, C. (2017). An overview of cochlear implant electrode array designs. Hearing Research, 356, 93–103. https://doi.org/10.1016/j.heares.2017.10.005[9]
- Optimal contact spacing offers the best of both worlds: mechanical flexibility and optimal channel spacing to reduce insertion force and avoid crosstalk from overlapping stimulation channels.Büchner, A., Illg, A., Majdani, O., & Lenarz, T. (2017). Investigation of the effect of cochlear implant electrode length on speech comprehension in quiet and noise compared with the results with users of electro-acoustic-stimulation, a retrospective analysis. PLoS ONE, 12(5), e0174900. https://doi.org/10.1371/journal.pone.0174900[6]Canfarotta, M. W., Dillon, M. T., Buss, E., Pillsbury, H. C., Brown, K. D., & O’Connell, B. P. (2020). Frequency-to-Place Mismatch: Characterizing Variability and the Influence on Speech Perception Outcomes in Cochlear Implant Recipients. Ear and Hearing, 41(5), 1349–1361. https://doi.org/10.1097/aud.0000000000000864[7]
- FLEX-Tip Technology helps ensure that the tapered, rounded electrode tip gently glides along the scala tympani during insertion to avoid damage to the basilar membrane and delicate structures.Dhanasingh, A., & Jolly, C. (2019). Review on cochlear implant electrode array tip fold-over and scalar deviation. Journal of Otology, 14(3), 94–100. https://doi.org/10.1016/j.joto.2019.01.002[10]Micuda, A., Li, H., Rask‐Andersen, H., Ladak, H. M., & Agrawal, S. K. (2024). Morphologic Analysis of the Scala Tympani Using Synchrotron: Implications for Cochlear Implantation. The Laryngoscope, 134(6), 2889–2897. https://doi.org/10.1002/lary.31263[20]
A systematic literature review, which analyzed data from 82 studies including a total of 8,603 ears implanted with a CI (4,869 with pre-shaped electrodes and 3,734 with straight electrodes) found that:
“The use of straight electrodes in robotic-assisted CI surgery and conventional CI surgery (and manual insertion) appears to be less traumatic to intracochlear structures compared with pre-shaped electrodes.”Heyning, P. V. de, Roland, P., Lassaletta, L., Agrawal, S., Atlas, M., Baumgartner, W.-D., Brown, K., Caversaccio, M., Dazert, S., Gstoettner, W., Hagen, R., Hagr, A., Jablonski, G. E., Kameswaran, M., Kuzovkov, V., Leinung, M., Li, Y., Loth, A., Magele, A., … Gavilan, J. (2022). Suitable Electrode Choice for Robotic-Assisted Cochlear Implant Surgery: A Systematic Literature Review of Manual Electrode Insertion Adverse Events. Frontiers in Surgery, 9, 823219. https://doi.org/10.3389/fsurg.2022.823219[15]
2. Pre-Operative Anatomy-Based Electrode Selection
Anatomy-based electrode selection, when an electrode array is selected based on the individual size of a cochlea, can provide pitch match closest to that cochlea’s specific natural tonotopic place-pitch map.Helpard, L., Li, H., Rohani, S. A., Zhu, N., Rask-Andersen, H., Agrawal, S., & Ladak, H. M. (2021). An Approach for Individualized Cochlear Frequency Mapping Determined From 3D Synchrotron Radiation Phase-Contrast Imaging. IEEE Transactions on Biomedical Engineering, 68(12), 3602–3611. https://doi.org/10.1109/tbme.2021.3080116[13]Li, H., Helpard, L., Ekeroot, J., Rohani, S. A., Zhu, N., Rask-Andersen, H., Ladak, H. M., & Agrawal, S. (2021). Three-dimensional tonotopic mapping of the human cochlea based on synchrotron radiation phase-contrast imaging. Scientific Reports, 11(1), 4437. https://doi.org/10.1038/s41598-021-83225-w[18]Li, H., Schart-Morén, N., Rohani, S. A., Ladak, H. M., Rask-Andersen, H., & Agrawal, S. (2019). Synchrotron Radiation-Based Reconstruction of the Human Spiral Ganglion: Implications for Cochlear Implantation. Ear & Hearing, 41(1), 173–181. https://doi.org/10.1097/aud.0000000000000738[19]
That is why MED-EL offers six FLEX arrays available in sizes from 20–34 mm so surgeons can choose the ideal array for each ear. Standard preoperative imaging and the electrode visualization feature in OTOPLAN* can help surgeons visualize how each electrode array would fit each patient’s unique cochlea with regards to insertion depth, frequency coverage, and scala tympani size.
3. Minimize Electrode Insertion Speed
Slow and consistent insertion has been shown to be associated with reduced force, contributing to reduced cochlear trauma.Aebischer, P., Anschuetz, L., Caversaccio, M., Mantokoudis, G., & Weder, S. (2024). Quantitative in-vitro assessment of a novel robot-assisted system for cochlear implant electrode insertion. International Journal of Computer Assisted Radiology and Surgery, 1–10. https://doi.org/10.1007/s11548-024-03276-y[2]Aebischer, P., Mantokoudis, G., Weder, S., Anschuetz, L., Caversaccio, M., & Wimmer, W. (2021). In-Vitro Study of Speed and Alignment Angle in Cochlear Implant Electrode Array Insertions. IEEE Transactions on Biomedical Engineering, 69(1), 129–137. https://doi.org/10.1109/tbme.2021.3088232[3]Hügl, S., Aldag, N., Lenarz, T., Rau, T. S., Becker, A., & Glasmacher, B. (2019). Identification of factors influencing insertion characteristics of cochlear implant electrode carriers. Current Directions in Biomedical Engineering, 5(1), 441–443. https://doi.org/10.1515/cdbme-2019-0111[16]Kontorinis, G., Lenarz, T., Stöver, T., & Paasche, G. (2011). Impact of the Insertion Speed of Cochlear Implant Electrodes on the Insertion Forces. Otology & Neurotology, 32(4), 565–570. https://doi.org/10.1097/mao.0b013e318219f6ac[17]Rajan, G. P., Kontorinis, G., & Kuthubutheen, J. (2012). The Effects of Insertion Speed on Inner Ear Function during Cochlear Implantation: A Comparison Study. Audiology and Neurotology, 18(1), 17–22. https://doi.org/10.1159/000342821[23]
“Robots can execute tasks beyond human dexterity and will probably pave the way to standardize residual hearing preservation and broadening the indication for electric-acoustic stimulation in the same ear with hybrid implants.”Abari, J., Heuninck, E., & Topsakal, V. (2024). Entirely robotic cochlear implant surgery. American Journal of Otolaryngology, 45(5), 104360. https://doi.org/10.1016/j.amjoto.2024.104360[1]
Precision cochlear implantation tools like OTODRIVE** and OTOARM can assist surgeons to ensure slow, steady, and precise movement during surgery.
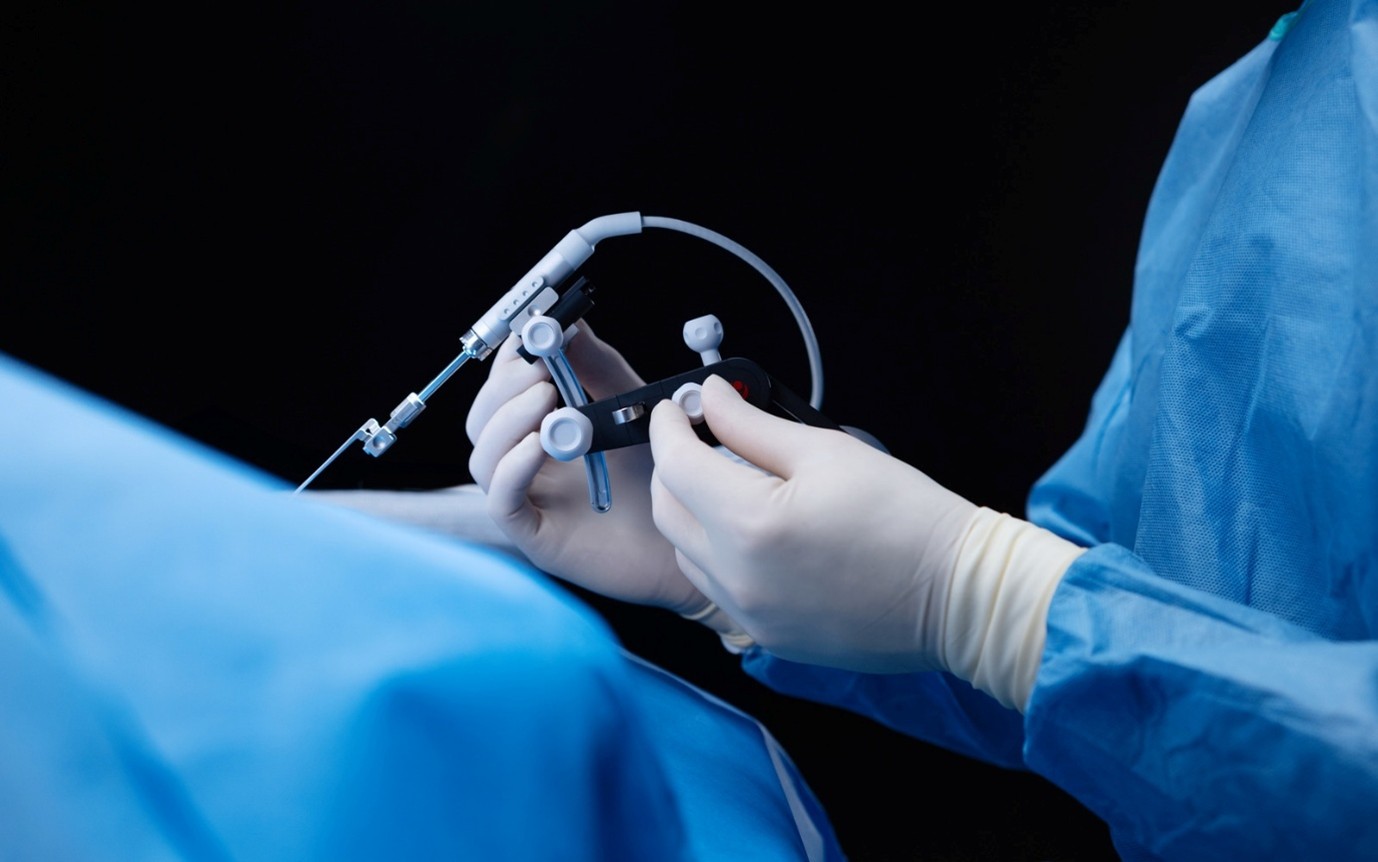
The OTODRIVE system can be used to maintain a constant insertion speed of 0.1 mm/s and minimize trauma with the “potential to enhance hearing and structural preservation outcomes.”Chen, J. M., Lin, V. Y. W., Le, T. N., Spiegel, J. L., Ungar, O. J., Bajin, M. D., Ladak, H. M., & Agrawal, S. (2025). Synchrotron‐Based Trauma Assessment of Robotic Electrode Insertions in Cochlear Implantation. The Laryngoscope. https://doi.org/10.1002/lary.32254[8]
When robot-assisted surgery was compared to manual insertion with nine different senior neurotologists, insertion with the OTODRIVE system with OTOARM “demonstrated a significantly slower and more controlled insertion speed (0.1 mm/sec) compared to manual insertion (0.66±0.31 mm/sec), which is crucial for minimizing intra-cochlear force and pressures.”Alhabib, S. F., Alzhrani, F., Alsanosi, A., Al-Amro, M., Alballaa, A., Shami, I., Hagr, A., Alahmadi, A., Sharif, T., Stichling, M., Matulic, M., Assadi, M. Z., Abdelsamad, Y., & Almuhawas, F. (2024). Robotic Versus Manual Electrode Insertion in Cochlear Implant Surgery: An Experimental Study. Clinical and Experimental Otorhinolaryngology, 18(1), 21–29. https://doi.org/10.21053/ceo.2024.00253[4]
Without major workflow disruptions, OTODRIVE and OTOARM can be integrated into the surgical routine, and “demonstrated a tendency toward improved hearing preservation and subjective benefit” for cochlear implant recipients; however, “larger, blinded and randomized trials are needed to determine the clinical value of [these motorized tools] for both objective and patient-reported outcomes.”Oetiker, Y., Aebischer, P., Caversaccio, M., Mantokoudis, G., & Weder, S. (2025). Hearing preservation outcomes with motorized cochlear implant electrode insertion: matched-cohort observations. Frontiers in Surgery, 12. https://doi.org/10.3389/fsurg.2025.1700744[26]
“What we found with our pre-clinical model is that, using the OTODRIVE system, we can really see a decline of pressure and force variation going into the cochlea.”
Prof. Dr. Stefan Weder
Co-Head of the Otology and Hearing Implant Center, University of Bern
While research is still ongoing, Prof. Dr. Weder has observed how OTODRIVE has the potential to help surgeons reduce insertion trauma.
Prof. Dr. Stefan Weder describes his experience using the OTODRIVE system.
Tools for Precision Surgery
Learn how OTODRIVE and OTOARM can help ensure precise, atraumatic movement and accurately position surgical tools.
Learn More4. Perform Round Window Insertion
With no need for an insertion stylet, FLEX arrays can be atraumatically inserted through the round window (RW) into the scala tympani, avoiding drilling trauma from a cochleostomy. A 2022 systematic literature review evaluated 3,797 CI patients:
“The RW approach resulted in a smaller … electrode-to-modiolus distance when compared to the cochleostomy approach. The RW approach (93.0%) led to statistically better hearing preservation than the cochleostomy approach (84.3%). The RW approach was also associated with better outcomes in terms of speech perception, ease of scala tympani insertion, and reduced scalar shift.”Avasarala, V. S., Jinka, S. K., Jeyakumar, A., & AVASARALA, V. S. (2022). Complications of Cochleostomy Versus Round Window Surgical Approaches: A Systematic Review and Meta-Analysis. Cureus, 14(5), e25451. https://doi.org/10.7759/cureus.25451[5]
Thus, a round window approach can contribute to maximizing hearing preservation for each patient. Plus, with FLEX electrodes there is no need for an insertion stiffener or stiff sheath to be inserted in the cochlea, resulting in minimal disturbance to the delicate structures at the base of the cochlea.
5. Reduce Inflammation With Corticosteroids
Steroid therapy continues to be one of the most practical and well-validated methods for mitigating intracochlear inflammation and fibrotic tissue response following cochlear implantation. By suppressing inflammatory cascades, corticosteroids can help preserve delicate cochlear structures, maintain neural survival, and protect residual acoustic hearing.
Both systemic and locally delivered steroids—whether administered intravenously, orally, or via intratympanic and intracochlear routes—have demonstrated measurable benefits in limiting fibrosis and maintaining lower postoperative impedance levels.
A major advancement in this field is the recent first-in-human clinical study, which evaluated a dexamethasone-eluting electrode array (the CIDEXEL system with a FLEX28-DEX array***).Prenzler, N., Salcher, R., Büchner, A., Warnecke, A., Kley, D., Batsoulis, C., Vormelcher, S., Mitterberger-Vogt, M., Morettini, S., Schilp, S., Hochmair, I., & Lenarz, T. (2025). Cochlear implantation with a dexamethasone-eluting electrode array: First-in-human safety and performance results. Hearing Research, 461, 109255. https://doi.org/10.1016/j.heares.2025.109255[22] In this prospective study, the investigators reported stable electrode impedances over time, encouraging hearing preservation outcomes—typically within 15 dB of preoperative thresholds, and no device or procedure-related serious adverse events.
Importantly, speech perception outcomes were comparable to standard electrode arrays, demonstrating that the integration of steroid delivery within the implant system does not compromise performance.
When viewed alongside foundational preclinical work Eshraghi, A. A., Wolfovitz, A., Yilmazer, R., Garnham, C., Yilmazer, A. B., Bas, E., Ashman, P., Roell, J., Bohorquez, J., Mittal, R., Hessler, R., Sieber, D., & Mittal, J. (2019). Otoprotection to Implanted Cochlea Exposed to Noise Trauma With Dexamethasone Eluting Electrode. Frontiers in Cellular Neuroscience, 13, 492. https://doi.org/10.3389/fncel.2019.00492[12]Plontke, S. K., & Salt, A. N. (2018). Local drug delivery to the inner ear: Principles, practice, and future challenges. Hearing Research, 368, 1–2. https://doi.org/10.1016/j.heares.2018.06.018[21] and earlier studies exploring sustained-release and topical dexamethasone application, these findings mark a pivotal step toward integrating pharmacologic therapy directly into implant design.
Collectively, the evidence reinforces that targeted steroid delivery—especially via drug-eluting electrodes—offers a powerful strategy to reduce insertion trauma, control inflammation, and enhance long-term hearing preservation in cochlear implantation.
MED-EL: The World Leader in Hearing Implant Innovation
With hundreds of research collaborations with clinicians around the world, we embrace diverse perspectives and expert knowledge to translate groundbreaking ideas into smart, life-changing innovations.
The Best Opportunity to Fully Benefit from Cochlear Implant Technology
Hearing and structure preservation are only part of the picture.
Learn More* OTOPLAN is a product of CASCINATION AG.
** CASCINATION AG is the legal manufacturer of OTODRIVE. MED-EL is the exclusive distributor of OTODRIVE. For compatible devices, refer to the relevant instructions for use. All devices are sold separately.
*** The CIDEXEL system with a FLEX28-DEX array is in development and is not yet commercially available.
References
-
[1]
Abari, J., Heuninck, E., & Topsakal, V. (2024). Entirely robotic cochlear implant surgery. American Journal of Otolaryngology, 45(5), 104360. https://doi.org/10.1016/j.amjoto.2024.104360
-
[2]
Aebischer, P., Anschuetz, L., Caversaccio, M., Mantokoudis, G., & Weder, S. (2024). Quantitative in-vitro assessment of a novel robot-assisted system for cochlear implant electrode insertion. International Journal of Computer Assisted Radiology and Surgery, 1–10. https://doi.org/10.1007/s11548-024-03276-y
-
[3]
Aebischer, P., Mantokoudis, G., Weder, S., Anschuetz, L., Caversaccio, M., & Wimmer, W. (2021). In-Vitro Study of Speed and Alignment Angle in Cochlear Implant Electrode Array Insertions. IEEE Transactions on Biomedical Engineering, 69(1), 129–137. https://doi.org/10.1109/tbme.2021.3088232
-
[4]
Alhabib, S. F., Alzhrani, F., Alsanosi, A., Al-Amro, M., Alballaa, A., Shami, I., Hagr, A., Alahmadi, A., Sharif, T., Stichling, M., Matulic, M., Assadi, M. Z., Abdelsamad, Y., & Almuhawas, F. (2024). Robotic Versus Manual Electrode Insertion in Cochlear Implant Surgery: An Experimental Study. Clinical and Experimental Otorhinolaryngology, 18(1), 21–29. https://doi.org/10.21053/ceo.2024.00253
-
[5]
Avasarala, V. S., Jinka, S. K., Jeyakumar, A., & AVASARALA, V. S. (2022). Complications of Cochleostomy Versus Round Window Surgical Approaches: A Systematic Review and Meta-Analysis. Cureus, 14(5), e25451. https://doi.org/10.7759/cureus.25451
-
[6]
Büchner, A., Illg, A., Majdani, O., & Lenarz, T. (2017). Investigation of the effect of cochlear implant electrode length on speech comprehension in quiet and noise compared with the results with users of electro-acoustic-stimulation, a retrospective analysis. PLoS ONE, 12(5), e0174900. https://doi.org/10.1371/journal.pone.0174900
-
[7]
Canfarotta, M. W., Dillon, M. T., Buss, E., Pillsbury, H. C., Brown, K. D., & O’Connell, B. P. (2020). Frequency-to-Place Mismatch: Characterizing Variability and the Influence on Speech Perception Outcomes in Cochlear Implant Recipients. Ear and Hearing, 41(5), 1349–1361. https://doi.org/10.1097/aud.0000000000000864
-
[8]
Chen, J. M., Lin, V. Y. W., Le, T. N., Spiegel, J. L., Ungar, O. J., Bajin, M. D., Ladak, H. M., & Agrawal, S. (2025). Synchrotron‐Based Trauma Assessment of Robotic Electrode Insertions in Cochlear Implantation. The Laryngoscope. https://doi.org/10.1002/lary.32254
-
[9]
Dhanasingh, A., & Jolly, C. (2017). An overview of cochlear implant electrode array designs. Hearing Research, 356, 93–103. https://doi.org/10.1016/j.heares.2017.10.005
-
[10]
Dhanasingh, A., & Jolly, C. (2019). Review on cochlear implant electrode array tip fold-over and scalar deviation. Journal of Otology, 14(3), 94–100. https://doi.org/10.1016/j.joto.2019.01.002
-
[11]
Dhanasingh, A., Nielsen, S. B., Beal, F., Schilp, S., Hessler, R., Jolly, C., & Hochmair, I. (2024). Cochlear implant electrode design for safe and effective treatment. Frontiers in Neurology, 15, 1348439. https://doi.org/10.3389/fneur.2024.1348439
-
[12]
Eshraghi, A. A., Wolfovitz, A., Yilmazer, R., Garnham, C., Yilmazer, A. B., Bas, E., Ashman, P., Roell, J., Bohorquez, J., Mittal, R., Hessler, R., Sieber, D., & Mittal, J. (2019). Otoprotection to Implanted Cochlea Exposed to Noise Trauma With Dexamethasone Eluting Electrode. Frontiers in Cellular Neuroscience, 13, 492. https://doi.org/10.3389/fncel.2019.00492
-
[13]
Helpard, L., Li, H., Rohani, S. A., Zhu, N., Rask-Andersen, H., Agrawal, S., & Ladak, H. M. (2021). An Approach for Individualized Cochlear Frequency Mapping Determined From 3D Synchrotron Radiation Phase-Contrast Imaging. IEEE Transactions on Biomedical Engineering, 68(12), 3602–3611. https://doi.org/10.1109/tbme.2021.3080116
-
[14]
Heyning, P. H. V. de, Dazert, S., Gavilan, J., Lassaletta, L., Lorens, A., Rajan, G. P., Skarzynski, H., Skarzynski, P. H., Tavora-Vieira, D., Topsakal, V., Usami, S., Rompaey, V. V., Weiss, N. M., & Polak, M. (2022). Systematic Literature Review of Hearing Preservation Rates in Cochlear Implantation Associated With Medium- and Longer-Length Flexible Lateral Wall Electrode Arrays. Frontiers in Surgery, 9, 893839. https://doi.org/10.3389/fsurg.2022.893839
-
[15]
Heyning, P. V. de, Roland, P., Lassaletta, L., Agrawal, S., Atlas, M., Baumgartner, W.-D., Brown, K., Caversaccio, M., Dazert, S., Gstoettner, W., Hagen, R., Hagr, A., Jablonski, G. E., Kameswaran, M., Kuzovkov, V., Leinung, M., Li, Y., Loth, A., Magele, A., … Gavilan, J. (2022). Suitable Electrode Choice for Robotic-Assisted Cochlear Implant Surgery: A Systematic Literature Review of Manual Electrode Insertion Adverse Events. Frontiers in Surgery, 9, 823219. https://doi.org/10.3389/fsurg.2022.823219
-
[16]
Hügl, S., Aldag, N., Lenarz, T., Rau, T. S., Becker, A., & Glasmacher, B. (2019). Identification of factors influencing insertion characteristics of cochlear implant electrode carriers. Current Directions in Biomedical Engineering, 5(1), 441–443. https://doi.org/10.1515/cdbme-2019-0111
-
[17]
Kontorinis, G., Lenarz, T., Stöver, T., & Paasche, G. (2011). Impact of the Insertion Speed of Cochlear Implant Electrodes on the Insertion Forces. Otology & Neurotology, 32(4), 565–570. https://doi.org/10.1097/mao.0b013e318219f6ac
-
[18]
Li, H., Helpard, L., Ekeroot, J., Rohani, S. A., Zhu, N., Rask-Andersen, H., Ladak, H. M., & Agrawal, S. (2021). Three-dimensional tonotopic mapping of the human cochlea based on synchrotron radiation phase-contrast imaging. Scientific Reports, 11(1), 4437. https://doi.org/10.1038/s41598-021-83225-w
-
[19]
Li, H., Schart-Morén, N., Rohani, S. A., Ladak, H. M., Rask-Andersen, H., & Agrawal, S. (2019). Synchrotron Radiation-Based Reconstruction of the Human Spiral Ganglion: Implications for Cochlear Implantation. Ear & Hearing, 41(1), 173–181. https://doi.org/10.1097/aud.0000000000000738
-
[20]
Micuda, A., Li, H., Rask‐Andersen, H., Ladak, H. M., & Agrawal, S. K. (2024). Morphologic Analysis of the Scala Tympani Using Synchrotron: Implications for Cochlear Implantation. The Laryngoscope, 134(6), 2889–2897. https://doi.org/10.1002/lary.31263
-
[21]
Plontke, S. K., & Salt, A. N. (2018). Local drug delivery to the inner ear: Principles, practice, and future challenges. Hearing Research, 368, 1–2. https://doi.org/10.1016/j.heares.2018.06.018
-
[22]
Prenzler, N., Salcher, R., Büchner, A., Warnecke, A., Kley, D., Batsoulis, C., Vormelcher, S., Mitterberger-Vogt, M., Morettini, S., Schilp, S., Hochmair, I., & Lenarz, T. (2025). Cochlear implantation with a dexamethasone-eluting electrode array: First-in-human safety and performance results. Hearing Research, 461, 109255. https://doi.org/10.1016/j.heares.2025.109255
-
[23]
Rajan, G. P., Kontorinis, G., & Kuthubutheen, J. (2012). The Effects of Insertion Speed on Inner Ear Function during Cochlear Implantation: A Comparison Study. Audiology and Neurotology, 18(1), 17–22. https://doi.org/10.1159/000342821
-
[24]
Schaefer, S., Sahwan, M., Metryka, A., Kluk, K., & Bruce, I. A. (2021). The benefits of preserving residual hearing following cochlear implantation: a systematic review. International Journal of Audiology, 60(8), 561–577. https://doi.org/10.1080/14992027.2020.1863484
-
[25]
US Food & Drug Administration. (2024). Premarket approval, MED-EL Cochlear Implant System. P000025/S129. https://www.accessdata.fda.gov/cdrh_docs/pdf/P000025S129B.pdf
-
[26]
Oetiker, Y., Aebischer, P., Caversaccio, M., Mantokoudis, G., & Weder, S. (2025). Hearing preservation outcomes with motorized cochlear implant electrode insertion: matched-cohort observations. Frontiers in Surgery, 12. https://doi.org/10.3389/fsurg.2025.1700744
References


Nathan Schackow, MA
Nathan is part of the MED-EL Professionals Blog editorial team. With a background as an English lecturer, Nathan has specialized in writing about innovations in hearing technology and care since 2020. His passion lies in translating complex science into clear, accessible communication that helps inform, inspire, and improve lives.
Stefano Morettini
Was this article helpful?
Thanks for your feedback.
Sign up for newsletter below for more.
Thanks for your feedback.
Please leave your message below.
CTA Form Success Message
Send us a message
Field is required
John Doe
Field is required
name@mail.com
Field is required
What do you think?
The content on this website is for general informational purposes only and should not be taken as medical advice. Please contact your doctor or hearing specialist to learn what type of hearing solution is suitable for your specific needs. Not all products, features, or indications shown are approved in all countries.


Nathan Schackow, MA
Nathan is part of the MED-EL Professionals Blog editorial team. With a background as an English lecturer, Nathan has specialized in writing about innovations in hearing technology and care since 2020. His passion lies in translating complex science into clear, accessible communication that helps inform, inspire, and improve lives.
Stefano Morettini

.png)