
Mario E. Zernotti
Published Aug 26, 2025
Managing Hearing Care in Children With Microtia and Atresia
Professor Mario Zernotti, our guest author, shares insights gained from nearly four decades of experience helping children with conductive hearing loss due to microtia and congenital aural atresia, which occurs most commonly in Andes Mountains of South America.

Since the beginning of my training in otorhinolaryngology, almost 40 years ago, it was very common to see pediatric patients with external malformations (microtias) and atresia of the external auditory canal (EAC). This high frequency was the main reason why, years later, our group and I became interested in external and middle ear malformations.
Medicine requires a constant search for information, and one of the many unanswered questions was the prevalence of congenital aural atresia (CAA) and microtia. To our surprise, we never found any literature on the subject until, fortunately, in 2014, the Pan American Health Organization provided us with the framework to study the prevalence of congenital aural atresia in Argentina.
We developed a protocol that, shortly after its implementation, encountered a serious problem: the variability of Argentina’s genetic structure. Residents of the northwest have a strong Amerindian genetic influence, while in the center or south, the European influence reaches almost 80% of the genetic map. This marked a clear difference or contrast, which led to our initial conviction about the genetic origin of CAA.
The Prevalence of Congenital Aural Atresia
“The region once comprising the ancient Inca Empire—the Andes Mountains from northern Argentina to Colombia—has the highest prevalence of congenital aural atresia in the world.”
Oral presentations at regional conferences reinforced this suspicion. These studies led to a publication in which we compared the worldwide prevalence: in many European papers the prevalence was 1 to 2 cases per 10,000 live births, while similar studies involving Hispanics and Native Americans had a prevalence of 4 to 5 cases per 10,000 live births.
In our study, we investigated 36,000 live births from three different regions and we found that the prevalence of CAA in children with Mendoza heritage (with high European genetic markers – Italian and Spanish) reached 1.8% per 10,000 live births, while in the northwest, in children with Jujuy heritage (with high levels of Aymara and Quechua genetic loads, indigenous peoples) it was 20.9 cases per 10,000 live births. This was the highest reported prevalence of congenital aural atresia in the world published and indexed on PubMed to date.Zernotti, M. E., Curet, C. A., Cortasa, S., Chiaraviglio, M., & Di Gregorio, M. F. (2019). Congenital Aural Atresia prevalence in the Argentinian population. Acta Otorrinolaringologica (English Edition), 70(1), 32-35. https://doi.org/10.1016/j.otoeng.2017.10.013[1]
This consolidated our research, and led us to engage with many patients with congenital aural atresia and microtia, as well as patient associations for them. Therefore, we have been pioneers in the placement of all available bone conduction implants—from percutaneous (BAHA Connect* and Ponto**) to the first passive bone conduction implants (Sophono and BAHA Attract*) and active intact skin implants since BONEBRIDGE was approved here in 2012.
Microtia and Atresia: Common Pathologies & Early Detection
It is important to remember that this unique patient population is always pediatric simply because congenital aural atresia and microtia are problems seen at birth that cause a lot of distress to parents. Today, these pathologies can be detected even during gestation with 3D or 4D ultrasounds. The association of CAA with other congenital malformations, including many syndromic conditions, is very common.
In our geographic region, it is common to observe syndromes associated with microtia and atresia. For example, one of the most frequent syndromes is Treacher-Collins, with a prevalence of 1 in every 25,000 to 50,000 live births, which often results in mandibular hypoplasia—a genetic disorder that most often affects the cheekbones, jaw, chin, and ears. The symptoms of mandibular hypoplasia, also known as micrognathia, typically include downward-slanting eyes, a very small jaw and chin, hearing loss, and vision loss. Some babies may be born with a hole in the roof of their mouth (cleft palate).
Another common syndromic condition is Goldenhar syndrome (1 in 25,000 live births), also known as oculoauriculovertebral dysplasia (OAV), which is a rare congenital disease characterized by abnormalities in the development of the eyes, ears, face, and spine, often affecting one side of the body. Treacher-Collins syndrome and Goldenhar syndrome are the two most common syndromes observed in patients with congenital aural atresia and microtia.
Audiological Assessment in Microtia & Aural Atresia
Regarding the audiological evaluation of these children, it is important to know that they cannot undergo neonatal hearing screening since otoacoustic emissions cannot be performed on them due to their lack of an external auditory canal. Furthermore, it is not until approximately two and a half years of age that we can perform classic speech and tone audiometry assessments, so it is necessary to evaluate these children early with auditory evoked potentials to confirm that their hearing loss is only conductive (because roughly 5% of them also have sensorineural hearing loss).
Pure Tone Audiometry: A Child With Microtia and Aural Atresia
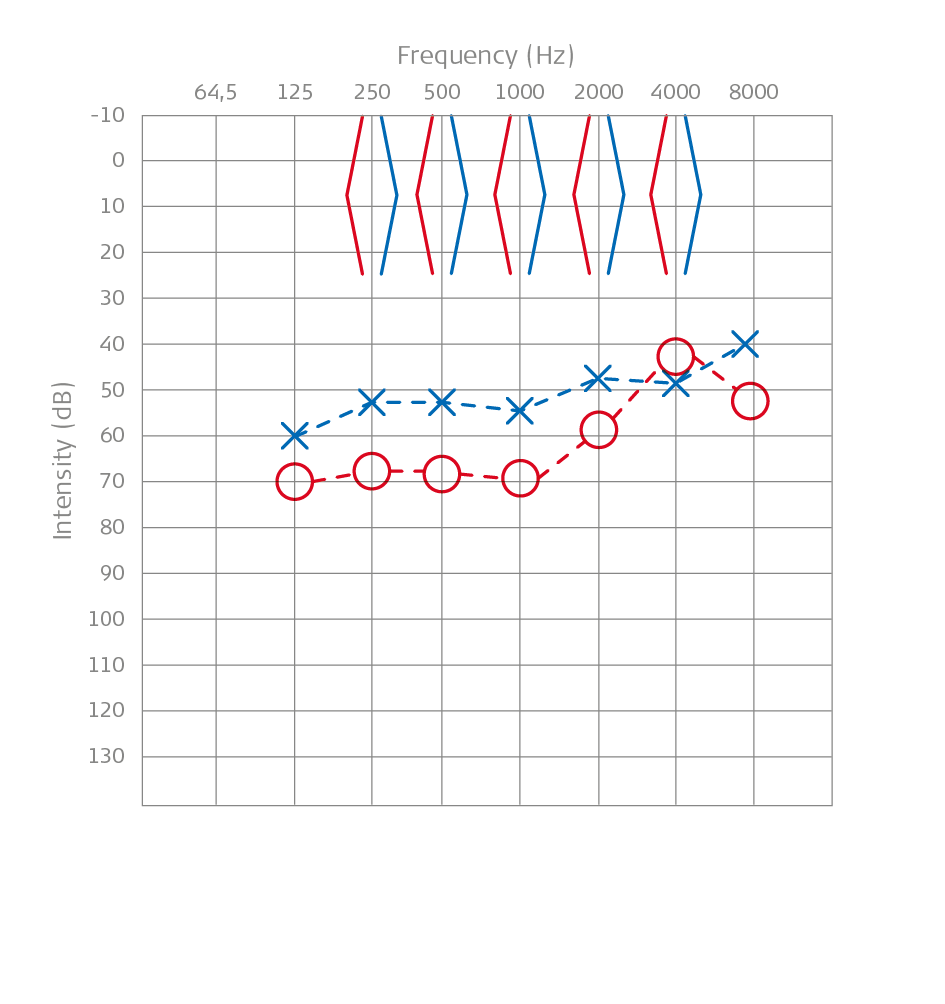
Above you can see the pure tone audiometry of a child, a boy named Maxi, with microtia and aural atresia. It shows responses to stimuli measured through the temporal bone and not with headphones.
The presence of conductive hearing loss with normal bone thresholds and air thresholds between 55 and 65 dB is the norm in these children. If we evaluate speech audiometry, discrimination rates are 100% at high intensities (80 dB), while at 65 dB, they reach approximately 40% word discrimination in open speech. These experiences led us to submit a treatment pathway for addressing children with microtia-atresia to the Ministry of Health in 2015.
Treatment Pathways for Addressing Children With Microtia-Atresia
Speech Audiometry: A Child With Microtia and Aural Atresia
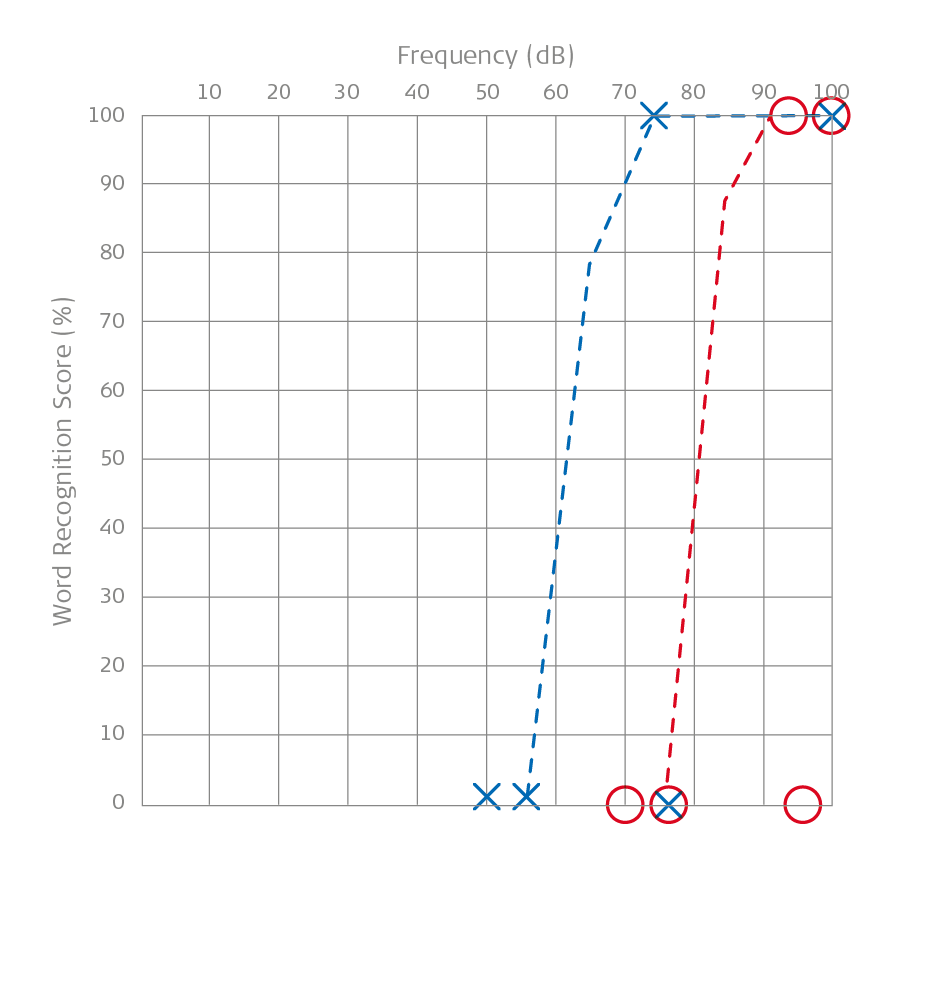
Above the speech audiometry of a boy named Maxi with microtia and aural atresia is shown. The right ear was measured with masking.
Treatment Pathways for Microtia and Atresia Patients
The first step we should take when we detect a newborn with microtia-atresia is to refer them to a pediatrician. There, they should evaluate the cardiac or cardiovascular system and the renal system, since the highest association in these patients is with malformations at this level. Once this point has been ruled out, we follow the treatment pathway.
We first perform screening with bone conduction auditory evoked potentials between one and three months of age. We do this while the child is physiologically asleep to avoid anesthesia or sedation, which is potentially harmful to cognition. We then begin early stimulation, and around 6 to 9 months, when the patient can hold their head up and sit, we equip them with any bone conduction device (soft band or adhesive).
Early Treatment Fosters Linguistic Development
Our clear preference is for ADHEAR (the non-surgical bone conduction adhesive device) since it does not apply pressure to the child’s mastoid and does not move as is the case with a soft band. With more than 30 dB of functional gain, we have seen very good outcomes with ADHEAR in congenital aural atresia cases in children.Zernotti, M. E., Alvarado, E., Zernotti, M., Claveria, N., & Di Gregorio, M. F. (2021). One-year follow-up in children with conductive hearing loss using ADHEAR. Audiology and Neurotology, 26(6), 435-444. https://doi.org/10.1159/000514087[2]
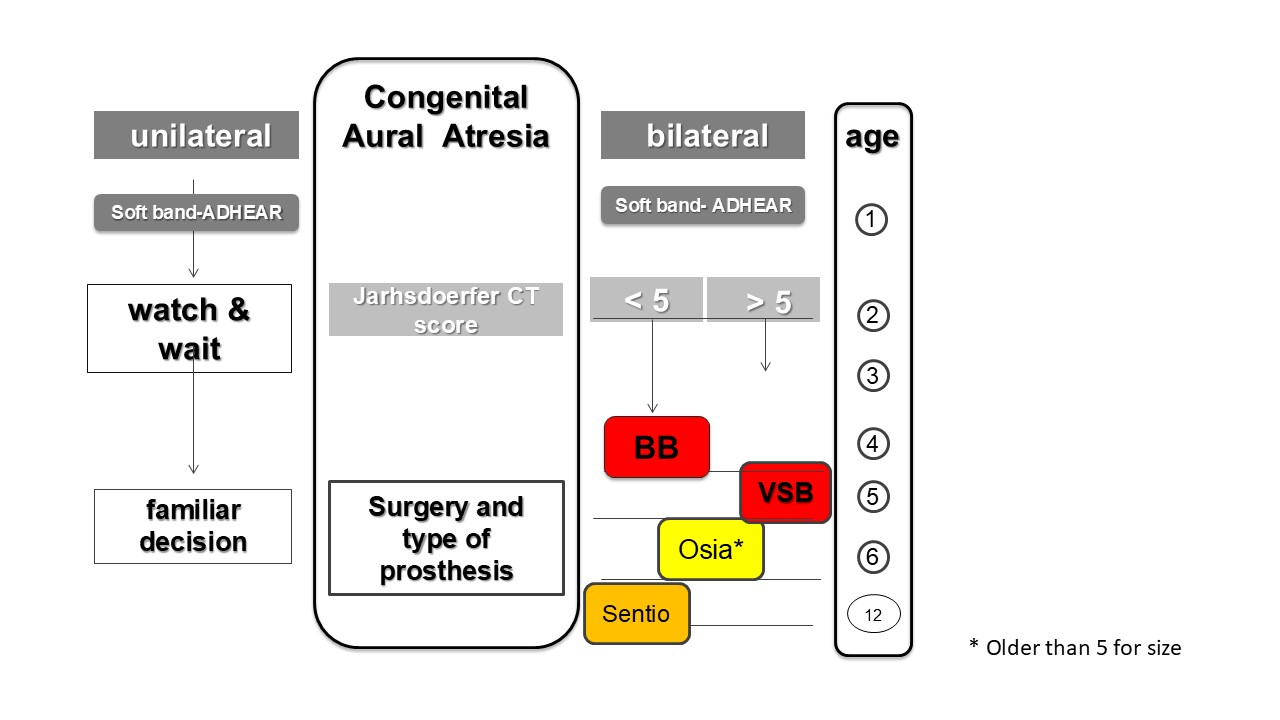
It should be noted that we use this protocol for children with unilateral or bilateral microtia and atresia, making no distinctions in this regard, to achieve the best possible binaural hearing in unilateral cases.
“It is abundantly clear that unilateral microtia and atresia brings with it many problems, such as impacting language acquisition. Although reversible, these problems must be treated. They also cause serious problems with early school enrollment, with some publications reporting a rate of grade repetition reaching 60%.”
Implantation Planning
We then perform imaging between 18 and 24 months: a computed tomography scan if the patient only has conductive hearing loss and an MRI if the patient’s hearing loss is mixed or sensorineural. We would like to make clear that the ideal pair of imaging is a CT and MRI scan, but this is not always available in emerging countries. If possible, we take advantage of a single use of anesthetic to perform both scans simultaneously.
The use of the adhesive device (or soft band) should be utilized until we can operate on the patient and place the bone conduction implantable prosthesis. We propose this for two main reasons. We have had extensive experience with atresioplasty, that is, surgery to drill a neocanal in these patients, and unfortunately, the audiological results have been poor; restenosis has been very frequent, and the presence of complications is very high. The literature supports our assertion, which is why, once the authorized age has been reached, we recommend surgery to implant active bone devices with intact skin.
The second reason is regulatory. The major regulatory agencies, the FDA, EMEA, and our ANMAT, authorize surgery starting at the age of 5. This is undoubtedly a totally arbitrary measure since age does not define anyone’s bone development.
“In our experience and that of many colleagues, it is essential to agree that the timing of implantation should be based strictly on anatomical rather than chronological criteria.”
Considerations on Bone Conduction Devices

Thus, we have implanted children under 5 years of age with excellent results and no complications, whose imaging studies showed sufficient bone to accommodate these devices. This is why imaging examinations and scans at this age are appropriate. At this point, the physician or audiologist colleague might ask why if we should continue with the adhesive prosthesis instead of subjecting the patient to surgery. The answer lies in the studies that demonstrate that BONEBRIDGE is superior to non-implantable devices. Our group compared ADHEAR to BONEBRIDGE and found that once implanted, the patient hears 10 to 15 dB more and discriminates 10% better.Di Gregorio, M. F., Der, C., Bravo-Torres, S., & Zernotti, M. E. (2024). Active Bone Conduction Implant and Adhesive Bone Conduction Device: A Comparison of Audiological Performance and Subjective Satisfaction. International Archives of Otorhinolaryngology, 28(02), e332-e338. https://doi.org/10.1055/s-0043-1777416[3]
Percutaneous vs. Transcutaneous & Active vs. Passive
Regarding percutaneous implants such as Baha* Connect or Ponto**, we can speak from our own experience, supported by numerous publications.
“Although they are more powerful, they present complications that easily exceed 30%, which leads to more problems than solutions, not to mention the fact that they are aesthetically unpleasant for users. So much so that the same companies that used percutaneous implants have developed protocols for their systematic replacement with intact-skin implants.”
We must then answer why an active implant with intact skin is better than a passive implant with intact skin. Passive devices are so named simply because they stimulate outside the skull, like non-surgical devices, and must overcome the resistance and impedance of soft tissues. This clearly makes them less potent. Many studies demonstrate that, like non-surgical devices, passive devices also have lower gains. We can affirm that this group of devices should no longer be included among the available prostheses.
BONEBRIDGE: Proven Reliability and Hearing Performance
Watch as Maxi, one of Prof. Mario Zernotti’s patients, describes his experience with BONEBRIDGE and SAMBA 2. This video was provided to MED-EL by Prof. Zernotti.
Finally, it is clear that the first option is a transcutaneous active implant, so we might ask why BONEBRIDGE and why not the new active devices for intact skin, such as Osia* and Sentio**?
We will try to explain our position. Here we can discuss several different aspects.
The length of time a product has been on the market is very important, and it varies greatly between them. BONEBRIDGE has been approved since 2012, Osia since 2019, and Sentio starting this year 2025.
“While this difference may seem trivial to some, it is key. Time allows a prosthesis to evolve based on the experience of users, audiologists, and surgeons, making it more reliable, and data corroborated by the FDA's MAUDE database. Time and data are needed to demonstrate the reliability and validity of a device.”
Hearing performance is crucial. In systematic reviews BONEBRIDGE provides more than 30 decibels of functional gain, reaching more than 40 decibels of functional gain in purely conductive hearing loss (auditory dysgenesis). Zernotti, M. E., Chiaraviglio, M. M., Mauricio, S. B., Tabernero, P. A., Zernotti, M., & Di Gregorio, M. F. (2019). Audiological outcomes in patients with congenital aural atresia implanted with transcutaneous active bone conduction hearing implant. International journal of pediatric otorhinolaryngology, 119, 54-58. https://doi.org/10.1016/j.ijporl.2019.01.016[4]
“Regarding discrimination, these patients go from a meager 30% or 40% discrimination to 98% to 100% discrimination. This translates into improved quality of life and improved academic, social, and work performance.”
Another important point of analysis is that it is aesthetically very wearable for children who will soon be adolescents and will face discrimination or bullying for wearing a prosthesis. This is not achieved with other devices.
Hearing Insights in Your Inbox
Get the latest research and resources to help people with every kind of hearing loss. Subscribe to the MED-EL Professionals Blog now.
Sign Up NowBONEBRIDGE: The Most Flexible Active BCI on the Market

“As surgeons, BONEBRIDGE offers us many more placement options, even in the most difficult anatomy—and it can do this with a truly low complication rate. This is a fundamental differential not achieved by other prostheses.”
Expanding on the topic of surgery, it is important to clarify that BONEBRIDGE has significant advantages over its competitors simply because the device is 4.5 mm deep and can be easily placed in three key positions (with the possibility of using the BCI 602 lifts to reduce depth to 3.5 mm). These are the mastoid (MARA), where it connects to the sinodural angle, where there is very good space and sufficient depth. When the mastoid is poorly developed or patients have undergone mastoid surgery for chronic otitis media, for example, the misnamed retrosigmoid approach can be used, which we call the posterior inferior approach (PIA). Finally, demonstrating the device’s versatility, the misnamed middle fossa position is used, since we don’t actually create a middle fossa by opening the dura mater to remove a tumor; that’s why we call it STELLA (Supra TemporaL Line Approach).Zernotti, M. E., & Gavilán, J. (2025). Who’s afraid of Dura Mater? Brazilian Journal of Otorhinolaryngology, 91, 101554. https://doi.org/10.1016/j.bjorl.2024.101554[6]
Optimizing BONEBRIDGE Placement With OTOPLAN
Only BONEBRIDGE has a titanium transition that can be moved 90° in either lateral direction, making it very easy to place. This allows the magnet portion to be positioned in the best area to fit the external portion (the audio processor) above the horizontal line that touches the upper part of the ear, while avoiding the shadow effect and placing the microphone in the best position for listening for the patient.
In addition, in this regard, BONEBRIDGE surgeons can benefit from the use of OTOPLAN, a software developed by MED-EL in collaboration with CASCINATION AG, that allows us to measure all anatomical parameters to choose the optimal position for each patient. This software works with CT images, allowing us to accurately determine whether the device will fit into the previously planned approach.
“OTOPLAN can be used to choose the best area to place the self-drilling screws based on where sound transmission will be most effective.”
Returning to the topic of patients with microtia and atresia, BONEBRIDGE also offers enormous advantages with OTOPLAN. We must recognize that children affected by microtia, and many parents, consider aesthetics to be very important. This is why reconstruction of the malformed auricle is crucial.
Reconstructive plastic surgeons have always complained that these devices prevent the creation of an ear, especially with the costal cartilage autograft technique (the most natural and least rejected by patients), since they affect the blood supply to the flaps near the auricle. We have developed a technique using the STELLA position with an incision just at the level of the BONEBRIDGE transition.Zernotti, M. E., Di Gregorio, M. F., & Zernotti, M. (2021). Alternative inverted middle fossa approach in bonebridge surgery. technique, results and complications. International Archives of Otorhinolaryngology, 25(03), e374-e378. https://doi.org/10.1055/s-0040-1715152[7]
This technique allows us, on the one hand, to minimize damage to the flaps that will be covered by the autologous cartilage graft (or implantable prostheses). On the other hand, it allows us to place the device as far as possible away from the area where the plastic surgery will be performed, without compromising the upper blood supply to the flap, which our plastic surgeon colleagues typically use for reconstruction.
BONEBRIDGE BCI 602
Learn how powerful direct-drive bone conduction, unmatched surgical flexibility, and healthy intact skin set BONEBRIDGE apart.
Discover MoreThank you to Mario E. Zernotti, Professor of Otorhinolaryngology at the Catholic University of Córdoba and the National University of Villa Maria as well as professor of Clinical Hearing at the National University of Córdoba in Argentina, for contributing this article filled his valuable insights.
For more on BONEBRIDGE reliability and safety compared to other bone conduction devices, a 2025 systematic review and meta-analysis on the safety profiles of bone-conduction hearing implants has been published here.
Views expressed are those of our guest author. Hearing professionals and clinicians should always refer to the relevant surgical guides and instructions for use for their country, which can be found here. Outcomes and results may vary.
* Baha and Osia are trademarks of Cochlear Ltd, Australia.
** Ponto and Sentio are trademarks of Oticon Medical Group, Denmark.
References
-
[1]
Zernotti, M. E., Curet, C. A., Cortasa, S., Chiaraviglio, M., & Di Gregorio, M. F. (2019). Congenital Aural Atresia prevalence in the Argentinian population. Acta Otorrinolaringologica (English Edition), 70(1), 32-35. https://doi.org/10.1016/j.otoeng.2017.10.013
-
[2]
Zernotti, M. E., Alvarado, E., Zernotti, M., Claveria, N., & Di Gregorio, M. F. (2021). One-year follow-up in children with conductive hearing loss using ADHEAR. Audiology and Neurotology, 26(6), 435-444. https://doi.org/10.1159/000514087
-
[3]
Di Gregorio, M. F., Der, C., Bravo-Torres, S., & Zernotti, M. E. (2024). Active Bone Conduction Implant and Adhesive Bone Conduction Device: A Comparison of Audiological Performance and Subjective Satisfaction. International Archives of Otorhinolaryngology, 28(02), e332-e338. https://doi.org/10.1055/s-0043-1777416
-
[4]
Zernotti, M. E., Chiaraviglio, M. M., Mauricio, S. B., Tabernero, P. A., Zernotti, M., & Di Gregorio, M. F. (2019). Audiological outcomes in patients with congenital aural atresia implanted with transcutaneous active bone conduction hearing implant. International journal of pediatric otorhinolaryngology, 119, 54-58. https://doi.org/10.1016/j.ijporl.2019.01.016
-
[5]
VIBRANT Technology Assessment Team. Systematic review and meta-analysis of audiological and patient-reported outcomes with the BONEBRIDGE active bone-conduction implant. MED-EL, 2021. Version 1.2 (May 2022). https://go.medel.pro/OutcomesBB
-
[6]
Zernotti, M. E., & Gavilán, J. (2025). Who’s afraid of Dura Mater? Brazilian Journal of Otorhinolaryngology, 91, 101554. https://doi.org/10.1016/j.bjorl.2024.101554
-
[7]
Zernotti, M. E., Di Gregorio, M. F., & Zernotti, M. (2021). Alternative inverted middle fossa approach in bonebridge surgery. technique, results and complications. International Archives of Otorhinolaryngology, 25(03), e374-e378. https://doi.org/10.1055/s-0040-1715152
References

Mario E. Zernotti
Mario E. Zernotti is a professor of otorhinolaryngology at the Catholic University of Córdoba and the National University of Villa Maria as well as professor of Clinical Hearing at the National University of Córdoba in Argentina. With over 30 years of experience in the field of ear surgery at the Sanatorium Allende, Prof. Zernotti is a pioneer of electric acoustic stimulation (EAS) and hearing preservation who implanted the first patients with EAS CIs in the Americas. In addition, his team, which has a comprehensive approach to atresia and microtia cases, was the first in the region to provide active middle ear implants and bone conduction prostheses. Prof. Zernotti is a member of the HEARRING Group, former president of GCIAN (Global Cochlear Implant Access Network) and President of the 8th World Congress on CI in Emerging Nations in 2024.
Was this article helpful?
Thanks for your feedback.
Sign up for newsletter below for more.
Thanks for your feedback.
Please leave your message below.
CTA Form Success Message
Send us a message
Field is required
John Doe
Field is required
name@mail.com
Field is required
What do you think?
The content on this website is for general informational purposes only and should not be taken as medical advice. Please contact your doctor or hearing specialist to learn what type of hearing solution is suitable for your specific needs. Not all products, features, or indications shown are approved in all countries.

Mario E. Zernotti
Mario E. Zernotti is a professor of otorhinolaryngology at the Catholic University of Córdoba and the National University of Villa Maria as well as professor of Clinical Hearing at the National University of Córdoba in Argentina. With over 30 years of experience in the field of ear surgery at the Sanatorium Allende, Prof. Zernotti is a pioneer of electric acoustic stimulation (EAS) and hearing preservation who implanted the first patients with EAS CIs in the Americas. In addition, his team, which has a comprehensive approach to atresia and microtia cases, was the first in the region to provide active middle ear implants and bone conduction prostheses. Prof. Zernotti is a member of the HEARRING Group, former president of GCIAN (Global Cochlear Implant Access Network) and President of the 8th World Congress on CI in Emerging Nations in 2024.

.png)



