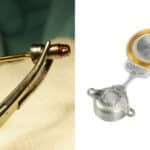
MED-EL’s Revolutionary S-Vector Magnet Technology

Introduced in early 2021, our S-Vector technology was the latest in a series of breakthroughs in magnet design that started decades ago at MED-EL. Since introducing our first multichannel cochlear implant in 1994, MED-EL has been recognized as the world leader in magnet technology. This is not only because our cochlear implants have provided outstanding MRI safety to recipients.* It is also thanks to the excellent track record of our devices: There has not been a single reported instance of magnet dislocation in MED-EL’s history. And that is only the beginning.
Our SYNCHRONY cochlear implant revolutionized hearing implant magnet design when we introduced it in 2014. It was the world’s first rotatable, self-aligning diametric cochlear implant magnet, which enabled 3.0 Tesla MRI scans without magnet removal.** This important milestone was reached thanks to research and development efforts focused on two key aspects of implant design: the type of magnet and secure fixation.
The Evolution of Cochlear Implant Magnets
Axial magnets, similar to standard magnets used on refrigerators, have basic north-south polarization. Because of the orientation of implants under the skin, magnetization is perpendicular to the skin’s surface. While these simple magnets can work well to hold audio processors in place, magnet torque or a “pulling” sensation can be experienced during an MRI if they are not secured by the implant’s design.
On the other hand, cochlear implants with diametric magnets have magnetization that is parallel to the surface of the skin. During an MRI, a diametric magnet is free to rotate, and does so continuously to align to the magnetic field as it changes. This rotation greatly reduces any magnetic torque, even in more powerful 3.0 Tesla scanners.[2]
What We Know About Magnet Fixation
The main design challenge to overcome was keeping the magnet securely in place while allowing for it still to be surgically removed if needed. Although a soft silicone pocket with a thin lip of silicone or silastic material can hold a magnet in place during everyday use and allow the magnet to be surgically removed for MRI scans, it can lead to issues when magnetic torque occurs during a scan and the magnet is not securely fixated.
An outstanding level of MRI safety is achieved when the magnets are securely embedded inside the implant so that they distribute torque force over a wider area to prevent any risk of the magnet dislocating during an MRI. Yet they must stay locked inside the coil while still being safely removable in case the image artifact needs to be reduced for scans near the implant.
For over 25 years, our earlier generations of multichannel cochlear implants have featured a fixed axial magnet securely embedded within the implant to allow for 1.5 Tesla scans without any risk of magnet dislocation. Our SYNCHRONY implant, which improved on the secure pocket designs of our previous implants, revolutionized cochlear implant design with a diametric magnet that can rotate to reduce torque force, allowing them to remain securely in place even during 3.0 Tesla MRI scans—with the additional advantage that magnet removal is optional to reduce the image artifact on brain scans near the implant.[1,3]
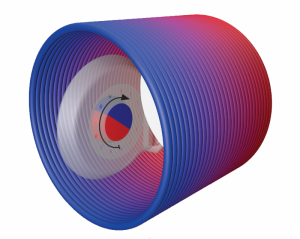
Inside of an MRI scanner, SYNCHRONY’s magnet rotates to align with the main static magnetic field.
While implants with axial magnets require the surgical removal of the magnet for every 3.0 Tesla MRI scan, the vast majority of 3.0 Tesla scans can be done leaving the diametric magnet in place (unless removal is required for diagnostic reasons).*
Overcoming the Next Challenge With S-Vector Magnet Technology
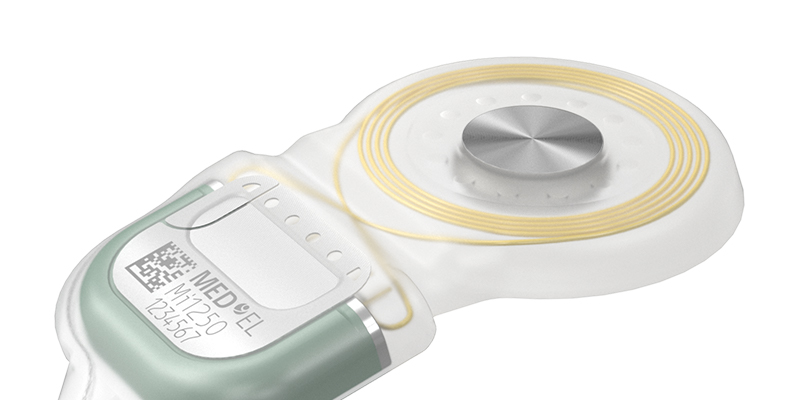
As the leader in implant magnet technology, we have now overcome the next big challenge in this area. Recipients, depending on anatomical conditions, sometimes face problems with the retention of their single-unit audio processor (SUP), worn off the ear. To overcome this problem and optimize retention for recipients while providing fitting flexibility to audiologists, MED-EL introduced our unique S-Vector magnet technology.
With our S-Vector magnet technology, we invented a second-generation diametric magnet for our SYNCHRONY 2 cochlear implant that is 25% stronger than its predecessor and provides all of the same benefits as the first-generation magnet.
The next benefit is that the S-Vector magnet technology provides recipients with an outstanding level of MRI safety expected from MED-EL implants. MED-EL is the first and only cochlear implant company to offer an MRI guarantee. This gives recipients greater peace of mind, knowing that if they need an MRI for any reason in the future, our MRI guarantee will cover cochlear implants with S-Vector magnets.
Great Protection. Guaranteed.
Because of our long and positive experience with MRIs and cochlear implants, we’re the only hearing implant company to offer a life-long MRI guarantee. We promise to replace the implant in the very unlikely event that it’s damaged during an MRI scan.*
- Valid for all MED-EL multichannel cochlear implants since 1994
- Life-long and worldwide
- The first and only to be offered by any hearing implant producer
Freedom for Recipients and Audiologists
Thanks to the increased magnetic pull strength of the S-Vector magnet, MED-EL cochlear implant recipients who take part in sports like sprinting or swimming—or have professions in which they often or suddenly move their heads—don’t have to worry about their SUP falling off.
Optimizing audio processor retention means that recipients and their audiologists now have more freedom to choose between a behind-the-ear audio processor or an SUP based on their own preferences, rather than their lifestyle or anatomical conditions such as skin flap thickness. This helps make it easier for cochlear implant recipients to adjust after activation, while providing audiologists with more flexibility during fittings.
Because the S-Vector magnet is 25% stronger, it not only provides recipients with increased magnetic force for optimized audio processor retention. It can also increase the margin of safety and, in some cases, give audiologists the opportunity to give recipients slightly weaker magnets during fittings. As a result, RONDO series audio processors can then be made even lighter.
Subscribe and Share
Looking for more information about the SYNCHRONY 2 implant or S-Vector magnet technology? Check out our SYNCHRONY 2 page for professionals.
* MED-EL cochlear implants since 1994—including the SYNCHRONY and SYNCHRONY 2 cochlear implants—are MR Conditional. Recipients with a SYNCHRONY or SYNCHRONY 2 cochlear implant may be safely MRI scanned at 0.2, 1.0, 1.5, and 3.0 Tesla following the conditions detailed in the medical procedures manual. The terms and conditions of our MRI guarantee can be found at https://go.medel.com/mri-guarantee-terms
** Unless required for diagnostic reasons.
References
[1] Helbig, S., Stöver, T., Burck, I., & Kramer, S. (2017). Cranial MRI in a young child with cochlear implants after bilateral magnet removal. International Journal Of Pediatric Otorhinolaryngology, 103, 1-4. https://doi.org/10.1016/j.ijporl.2017.09.028
[2] Todt, I., Tittel, A., Ernst, A., Mittmann, P., & Mutze, S. (2017). Pain Free 3 T MRI Scans in Cochlear Implantees. Otology & Neurotology, 38(10), e401-e404. https://doi.org/10.1097/mao.0000000000001569
[3] Wagner, F., Wimmer, W., Leidolt, L., Vischer, M., Weder, S., & Wiest, R. et al. (2015). Significant Artifact Reduction at 1.5T and 3T MRI by the Use of a Cochlear Implant with Removable Magnet: An Experimental Human Cadaver Study. PLOS ONE, 10(7), e0132483. https://doi.org/10.1371/journal.pone.0132483
CTA Form Success Message
Send us a message
Field is required
John Doe
Field is required
name@mail.com
Field is required
What do you think?
The content on this website is for general informational purposes only and should not be taken as medical advice. Please contact your doctor or hearing specialist to learn what type of hearing solution is suitable for your specific needs. Not all products, features, or indications shown are approved in all countries.