MED-EL
Published Dec 20, 2018
HEARO Robotic Cochlear Implantation: Prof. Vedat Topsakal with Prof. Paul Van de Heyning
The dawn of a new era in cochlear implant surgery: On December 10th, Professor Vedat Topsakal performed the first HEARO robotic cochlear implantation procedure on a patient at UZA Antwerp University Hospital.
HEARO®, the world’s first cochlear implant surgical robot, has been developed by CAScination AG, together with MED-EL. This landmark achievement is the result of years of dedicated research and development across multiple disciplines. In 2016, Prof. Marco Caversaccio of Insel Hospital, Bern, proved that it was possible to safely pass the facial recess and perform a robotic access to the middle ear cavity using this technology.
Now, Prof. Topsakal has proven that it is possible to robotically drill an access to the inner ear and safely create a channel all the way to the round window of the cochlea. With OTOPLAN, you can calculate the ideal trajectory for each patient for an optimal approach angle for insertion into the cochlea. Aligning the insertion trajectory to the natural cochlear anatomy enables a smooth insertion of the electrode array.
HEARO creates this straight trajectory from the mastoid surface to the scala tympani at the ideal approach angle for entering the cochlea through the round window, allowing the surgeon to gently insert the flexible electrode array directly into the scala tympani.
The HEARO procedure replaces the traditional mastoidectomy, posterior tympanotomy, and removal of the bony overhang with a precisely drilled channel directly to the round window of the cochlea. The entire procedure is planned by the surgeon pre-operatively using OTOPLAN® software.
Can’t See This Video?
Having problems viewing this video? Watch it on YouTube.
HEARO uses multiple integrated safety measures to minimize the risk of structural or heat damage to the facial nerve, chorda tympani, and other critical anatomical structures. Active safety measures include continuous neuromonitoring, interoperative imaging-based verification of safety margins with OTOPLAN, and real-time responsive torque-force monitoring while drilling. Additional passive safety elements include micro-precision image guidance and specialized robotic drilling technology developed to minimize any heat generation.
When approaching and passing through the facial recess, HEARO provides advanced facial nerve monitoring to actively map the safety margins to the facial nerve, fully independent of image guidance.
Today, we’re excited to hear from Prof. Vedat Topsakal and Prof. Paul Van de Heyning. Let’s go to them now to learn their insights on the procedure, on tablet-based surgical planning, the role of the surgeon with HEARO, and the future of HEARO and robotics in otology.
Prof. dr. Vedat Topsakal
When you think of a robot, everybody immediately thinks a robot can be consistent, it does every time the same thing. Proving that the system is consistent and safe in terms of surgical safety, I think that’s the first step.
Pioneers have already proven that the mastoid, the first exposure that you have to have for this surgery, has been overcome. This robotic technology can overcome the facial nerve and bypass the facial recess. You have to have a window of 2.3 millimeters to come through with this robot just to continue to the middle ear and this was proven already.
We worked for more than a year from that point to advance two more millimeters just to have access to the inner ear, and that two millimeters took at least one and a half years of study.
This is a clinical trial and we report our result in the most professional way. We encourage people to doubt our results, and repeat our experiments, this is how science advances. That’s good science, because you have to have repeatable science; that is just basically proving that somebody else did it.
We repeated the result of the Bern trial, and we went two millimeters beyond—we safely opened the inner ear and we smoothly placed the cochlear implant.
And HEARO performed so well, and I might perhaps even call it beginner’s luck, but we had such a nice insertion angle, which I have really never seen in my personal series, and the electrode was covering the lateral wall perfectly as we aimed.
HEARO Procedure
I think the most impressive trait is the accuracy of this system, it’s less than a thickness of your hair. So the precision is there and the precision is needed because we have to go through a facial recess—basically you have to pass over the facial nerve and underneath the chorda tympani.
That’s about 2.3–2.5 mm and then you have to make an opening of 1.0 mm, and through that channel you have to manage to place an array into the cochlea.
When you think of the ideal line to access the cochlea, it’s a bit striking that the ideal trajectory goes through the facial nerve. Of course, the facial nerve is one of the most important structures not to harm because it is such a burden to have a paralysis of your mouth and your eye muscles. Obviously this we can not do, but we have to go over it and see how the technology fits.
You can give the facial nerve a stimulus (current) to see how close you are from it. And this is what HEARO does, also next to the imaging that we do in intraoperatively, to give the feedback that we need.
And I think it’s becoming really technical, but the safety margins we apply now is within two or three times the accuracy of the system, to be on the safest side possible at the moment.
And by matters of a joke, I say the surgery is done on a tablet, because when you planned this trajectory, the decision-making part of surgery is already done. The HEARO procedure only performs or executes the trajectory that you’ve planned with such a precision; I can rely on it.
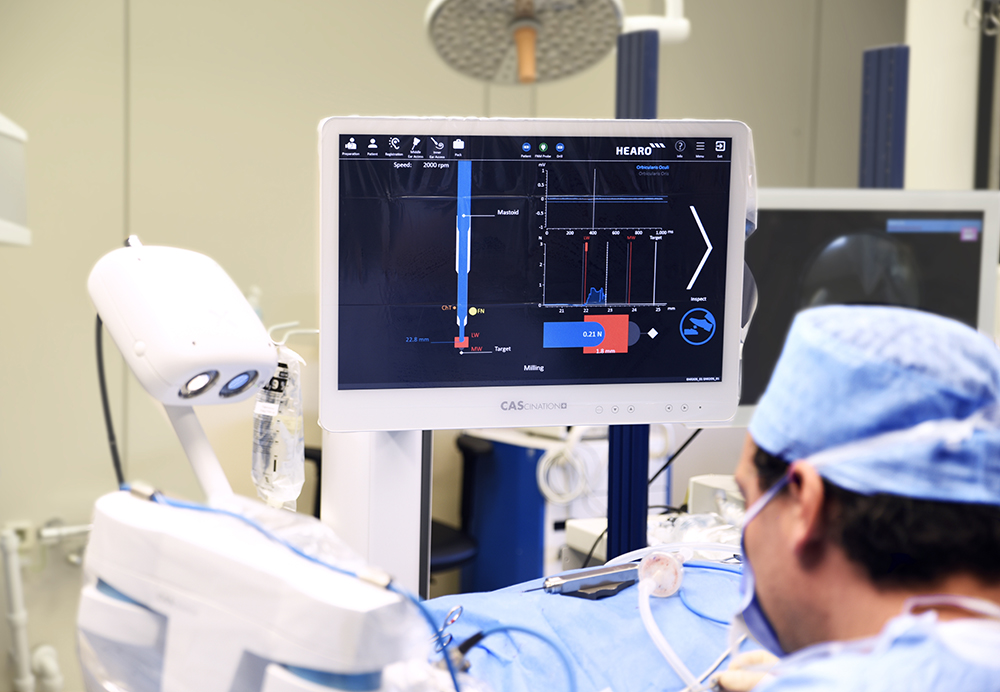 HEARO inner ear access: Automatically drilling the bony overhang of the cochlea using image guidance and drilling-force monitoring to safely access the round window.
HEARO inner ear access: Automatically drilling the bony overhang of the cochlea using image guidance and drilling-force monitoring to safely access the round window.
But it’s not a threat to the surgeon at all. This device will not work without a surgeon. It’s like an electric bike—it’s an electric boost, but if you don’t pedal as a cyclist, it’s not going to advance.
You know when you get a new microscope and we have a 3D microscope, everybody’s really excited and you wear these 3D glasses and it makes it look like a cinema, everybody’s excited. But once you say it’s a robot, people get threatened by, “Oh it might be something that will take over my job.”
I don’t feel like that at all. It is a new technology and I really feel it’s our responsibility to get acquainted with it, to learn how to use it, to know its benefits and maybe its disadvantages also. This is this is my task to study and to share with my colleagues.
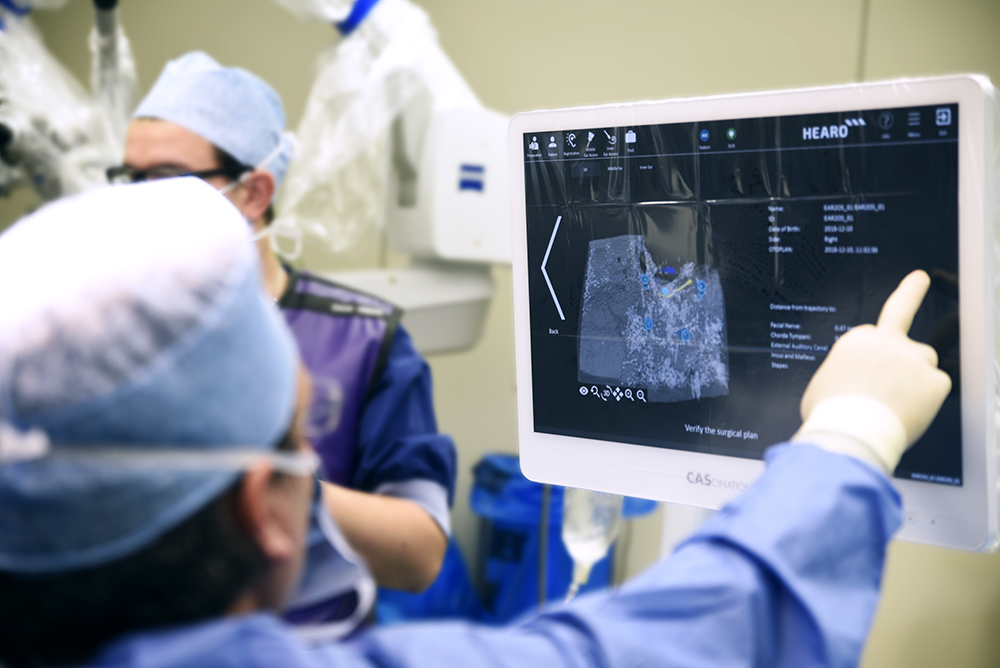 HEARO allows the surgeon to be in full control using an integrated touch-screen interface and step-by-step guidance.
HEARO allows the surgeon to be in full control using an integrated touch-screen interface and step-by-step guidance.
Surgeons will always be needed, and it will always be human-to-human contact. The robot is just a tool and you should use it, you should learn how to use it, just to aim for better results for your patients.
If you’re in need of a cochlear implantation because you have a hearing disorder and you have a social burden with communication, you need to have a cochlear implantation. It doesn’t matter actually, with or without the robot, with or without technology, or with a classically schooled surgeon. You just basically go to a physician, and there is a patient–physician trust or connection, and that’s the most precious thing to really care for together.
Patient-Specific Planning
As a surgeon, every step we do in the surgery is really preparing for that last moment when the array is going into the cochlea. And that’s really the moment where you would like to have the positioning of that array that you planned already in your mind.
As a research group or as a cochlear implant group, we truly believe that the full length of the cochlear duct should be used. Why leave half of the cochlea unused whereas nature uses it as a whole?
You have to try to cover the whole cochlea, because there’s a lot of nerve tissue there that can be benefiting from the electrode, of the electrophysiology or the technology. And we truly believe that you have to have an implant design that is soft, but still gets the job done. It should not harm when inserting the cochlea.
That’s the way we think, the way we feel about customizing the length of the array to the length of the cochlear duct. This was maybe a non-issue 5, 10, or 15 years ago when it was almost a miracle that when an electrode went in, patients could hear and understand speech. You cannot blame the pioneers putting an electrode in and trying to make the patient hear. But nowadays we are beyond that point.
Today we are challenging much more than that. It’s not only about hearing something, it’s about hearing in a noisy environment; it’s about hearing comfort; it’s about maybe music awareness or appreciation.
Based on the cochlear duct length measurements or predictions used with OTOPLAN planning software, we’ve chosen the 28 mm FLEX electrode from MED-EL. Why? Because we feel it is a very nice electrode that covers the lateral wall. It’s a flexible electrode, it’s not harming any intracochlear tissues.
OTOPLAN is basically a portable medium where you can walk through your hospital, to your OR to your patient and show them the scan, their own scan, and explain to them in a personalized way that this is what we’re going to do with the surgery. This is your anatomy, this is your cochlea, and this is the required implant—or we feel this is the best implant for you.
First patient or case I studied in OTOPLAN took me 45 minutes, maybe an hour. Then it was like a Gameboy in your hand. I was just playing with it in front of the telly, in the kitchen, everywhere. After a couple of days already I was really confident with it.
And nowadays it would not take me more than five minutes to measure the cochlear duct length to see what I find is the appropriate electrode to choose for that patient, and just to send a report to our digital patient file or even have a print of that or even put in a PowerPoint.
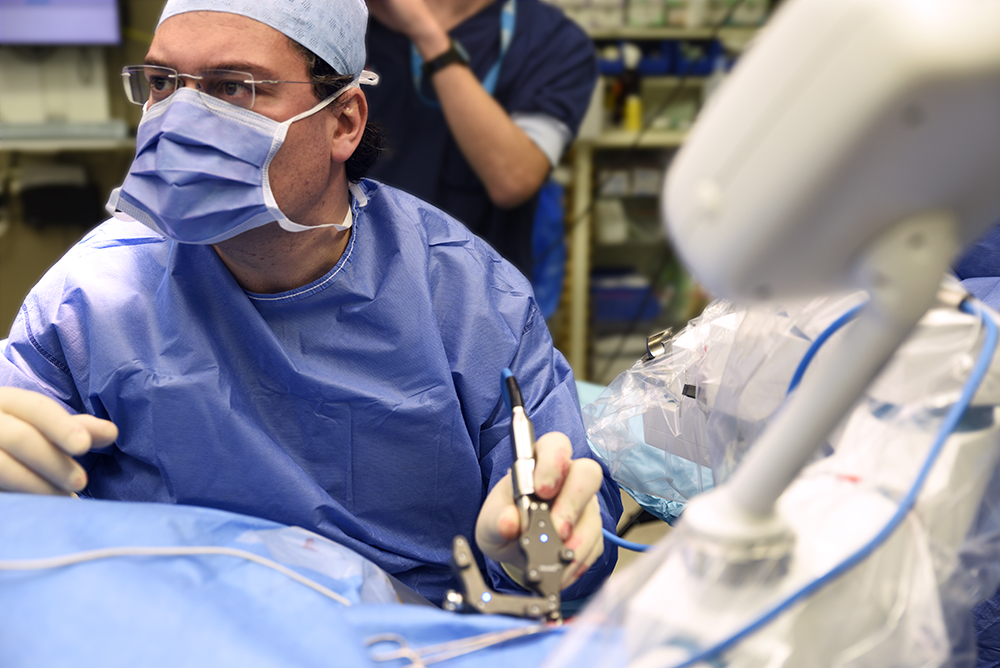 Prof. Topsakal performing the fiducial marker registration step. HEARO uses precise optical tracking to compensate for any micro-movement.
Prof. Topsakal performing the fiducial marker registration step. HEARO uses precise optical tracking to compensate for any micro-movement.
What I really liked about today is it went well for my patient. Using technology in medicine is not to help doctors be more efficient or whatsoever, it’s really to help patients. I think this patient is really mature, strong minded and open-minded, and also courageous enough to try to help science.
He had been wearing his hearing aids for many, many years and he came to a point that it just was not helpful enough. He trusted me to do the surgery in the first place, anyhow in a classical way. He said, “Well, I completely trust you” and I thank him very, very much for that.
And in a way, he has chosen to give this decision of his to the trust he gives us. I think the first patients with this technology, with HEARO robot, the patients with this robotic surgery, are very, very courageous and they want to contribute to science.
But once a certain amount of patients have had the courage to do this, I truly believe that there will be people coming to us and saying, “Oh I need mine robotic because it’s better and more precise.”
 Success: Prof. Topsakal and the UZA team, together with the CAScination and MED-EL support team.
Success: Prof. Topsakal and the UZA team, together with the CAScination and MED-EL support team.
What I really like is the intense work with engineers, we give immediate feedback to each other always to improve the product in the system, and it’s a fun way of working with engineers who think differently than surgeons. Of course, Prof. Stefan Weber (ARTORG) is following our every step, but the close-contact discussions with Marco Matulic (CAScination) and Masoud Zoka Assadi (MED-EL), their inexhaustible enthusiasm was for me a guarantee to continue with this project.
After the surgery: I felt the same as I always do. You have a surgical procedure that you’ve trained for, it’s just a protocol that you have to run down. So, I felt well-prepared and I felt some excitement because nobody else had done this procedure. But as being in training, we’re always used to doing a procedure for the first time.
Having practiced this procedure over and over, I feel very happy that it has proven itself worthy, so it’s just the beginning today. And we have accomplished not so much—we have shown what we already thought was possible, so we have to prove now that this is the way to go in cochlear implant surgery.
Prof. dr. Paul Van de Heyning
First of all, we are very relieved and excited that the surgery went well, and that the patient has a cochlear implantation with full insertion of all electrodes, and that the patient had not had any disadvantage of being the first patient in this type of high-tech complicated surgery.
It was a milestone. I feel very pleased with the fact that we could achieve these results, but to be honest, before the surgery I was quite confident that the surgery would go well. I was quite confident with the amount of preparation, careful preparation, the amount of energy of the whole team, the preparation of the year, the preparation of the last months, and over the last weeks.
 Prof. Van de Heyning with Prof. Topsakal and the surgical support team from UZA, CAScination, and MED-EL.
Prof. Van de Heyning with Prof. Topsakal and the surgical support team from UZA, CAScination, and MED-EL.
About one year ago, at a meeting in Perth, when it was presented to our colleague Professor Vedat Topsakal, and it caught his attention and he said, “We must do that in Antwerp.” It made things evolve quite rapidly, much more. I think it would not have been the case unless he was so determined that we would be able to do it.
And it was only possible because of the application and the existence and availability of all these tools, together with a very intensive way of training in every step, up to the fact that the temporal bone of the anatomy of this patient was first mimicked in 3D printing, and then also testing out it in the particular anatomy of this patient—that I was quite confident.
At the end the patient got a perfect cochlear implantation. And you can look upon that like sending a satellite to Mars. And it is, at very end, that you know whether, “Okay we landed correctly or not,” and this gives a very, very big satisfaction of course.
From OTOPLAN to HEARO
You’re not just entering in the OR and saying, “Today we are going to do a robotic insertion of cochlear implantation.” That’s not the way that it works. And so you evolve step-by-step.
And so first it was a kind of natural evolution—first working OTOPLAN and using it for completely different purposes but getting acquainted with the software and with the reliability and finding our way using this software.
We immediately saw already the advantages in using OTOPLAN on itself. OTOPLAN is a very friendly-to-use program; load in the raw data from the radiology and the surgeon is able to handle them. You can precisely define with this program what is the necessary length to cover in this cochlea, and so this gives us a big help to make the appropriate choice of electrodes.
It works first to say, oh, being astonished now we have a software that as surgeons we can use, we can handle our own images, we can look at different angles, and that’s the first step.
And the next step is we can use it to define better cochlear size and to define what is the exact electrode we need for that particular purpose.
When we make a choice for patient with hearing loss, we want a coverage of all the frequencies—the high-pitch, low pitch, and especially the low pitch. And in these patients, we want our electrode to go into the second turn in order to stimulate also those regions of the low frequencies. For instance, to make the difference between male and female voice, to understand how to appreciate prosody, music, better speech in noise, you need hearing in low frequencies.
And then your next step is, “Oh wait, maybe it can help us to define what is the angle of insertion of it.” And step by step, we have your experience growth, but also the expectations grow.
And in a quite natural way, you get into the idea of, “Maybe we can use this robot in a surgical patient,” and it is not some experiment anymore in a lab on artificial temporal bones or on real temporal bones, but we see the reality to be able to do it.
Robotic Surgery & Otology
I’m convinced when we start with robotic surgery in ear surgery that many of our colleagues will say we do not need it. It’s just like the beginning of the cochlear implantation. As long as we could not do a cochlear implantation, there were no people thinking about what to do with people that develop a very severe hearing loss.
We had sign language, all these important things for people, but from the moment that cochlear implantation was possible, a lot of science developed around it, because now it had a point, it had a purpose.
I witnessed the first discussions of cochlear implantation many years ago in 1983, and everything that was said that could not be done, “This is impossible, we will never be able to do that,” meanwhile has been done.
It is the same with the robot now. It will lead to a boost of new ideas. As long as something cannot be done, then there’s no point of developing new applications or new benefits.
Now that we can do it, then you will see that in a short time many new ideas will develop due to the fact that there is a finality in it.
One of the greatest challenges with robotic surgery in otology is the precision needed, because of the intricate scale of the structures.
Robotic surgery is a tool that now exists, and that shows that it has the precision to be applicable in otological surgery, but the way has been made by many other robotic surgeons.
The only reason why this was not the case in otologic surgery is because in otology, everything, every technology is pushed at its limits. Up to now, it was unable to gain enough precision, the precision was much less than you could do with your hands, so it was self-evident that robotic surgery in ear surgery had no place.
And so also this surgery, not too long ago, one could hardly imagine that it would be possible to do this type of surgery with a precision of 0.1 millimeter or beyond that, below that. And today we proved that it is possible and so this is really a milestone.
Going Beyond
The margins that we take in our regular surgery for the facial nerve and the chorda tympani are much smaller than we actually do now with the HEARO. We can discuss on facial nerve, we can discuss on chorda tympani, but regular good surgeons will not harm a facial nerve and the majority, not all, will not harm the chorda tympani.
But the future, or the advantage of the future will not just be “Oh, we can do it maybe safer,” because good surgeons operate in a safe way. Rather, it is a tool that allows us to do things that we could not do at this moment.
And it is not just one tool, it is a complete set of tools that have been developed. And maybe people are impressed by the robotic arm doing the drilling, but drilling is only possible if we could do high precision scans which are more precise than most CT scans.
During the surgery, when we could do new ways of endoscopy and looking through the channel to the place where we went for doing the last part of the insertion of the electrodes. To have a new developed system of facial nerve monitoring, to have a telemetry system of monitoring how far the electrode advances in the cochlea. This idea of robotic surgery pushed everyone to develop a complete set of new technology.
And now already in the preparation of HEARO surgery, we notice that when we were designing the approach of the electrode, that it was quite different to what we are doing with our hands in normal surgery. So just working with it influenced already the way that we are doing our regular surgery.
But we feel now, already from this first case, and when we saw at the scan postoperatively that it was so perfectly placed, that we think that we will be able to prove next year that placement with the robot can be done in a better way, in a more appropriate way than we do it by hand.
And this is a turning point, because now we are able to do it and to get the precision which is at least at what we can do ultimately, and that the future will show it is able to things we cannot do.
So it will gain its place. There’s no question whether it is the case, it’s only the question, “How fast will this evolve?”
Now having done one, it will go on. There’s no way back.
Fantastic insights—our thanks to Prof. Topsakal and Prof. Van de Heyning for sharing their experiences, we’re excited to hear more about HEARO robotic surgery and cochlear implant in the near future!
*Not all products, indications, and features shown are available in all areas. Please contact your local MED-EL representative for more information.
**HEARO and OTOPLAN are produced by CAScination AG.
MED-EL
Was this article helpful?
Thanks for your feedback.
Sign up for newsletter below for more.
Thanks for your feedback.
Please leave your message below.
CTA Form Success Message
Send us a message
Field is required
John Doe
Field is required
name@mail.com
Field is required
What do you think?
The content on this website is for general informational purposes only and should not be taken as medical advice. Please contact your doctor or hearing specialist to learn what type of hearing solution is suitable for your specific needs. Not all products, features, or indications shown are approved in all countries.
MED-EL



