MED-EL
Published May 16, 2018
A Deaf Ear is Not a Dead Ear: Looking Inside the Cochlea With Prof. Helge Rask-Andersen

Today, we are sharing a fascinating contribution from Professor Helge Rask-Andersen, one of the leading researchers in cochlear anatomy and physiology.
Prof. Rask-Andersen is a specialist in ENT & Audiology, an anatomist and electron microscopist, and he is a senior Professor in experimental Otology at the Uppsala University and the Academic Hospital in Uppsala, Sweden. He currently runs the Research Department at the Academic Hospital.
In this article, Prof. Rask-Andersen explains the intricate functions of the cochlea and why structure preservation should be a priority for every cochlear implant surgery—even if there is no residual hearing. He shows why damage to the delicate cochlear structures can have such an adverse effect on the health of the underlying neural structures, and the impact this can have on outcomes with a cochlear implant.
Let’s turn over to Prof. Rask-Andersen and hear his perspective on the importance of respecting and protecting the structures of every cochlea.
The Importance of Structure Preservation in Cochlear Implantation
During my medical studies, I learned electron microscopy and I have been devoted to human inner ear anatomy ever since. My first interest was Meniere’s disease and endolymphatic sac. I have been examining the human cochlea for 30 years now. Since I performed skull base surgery with my teacher, the extremely skilled surgeon Dr. Anders Kinnefors, I have had a deep interest in cochlear and auditory nerve anatomy. At first it was more academic, but with CI it turned into applied anatomy with clinical orientations.
In my research, my most interesting finding was the unique structure and the remarkable conservation of the human auditory nerve. This did not adhere with animal studies. I found that the human auditory nerve cell soma are unmyelinated, denied by many experimentalists. This was not a new finding but together with molecular work, it seemed to explain why the human nerve behaves differently and persists after hair cell loss and deafness; a blessing for the deaf and implanted.
Incredible Intricacy
There are scant and tremendously vulnerable tissue membranes in the inner ear—the Reissner´s membrane is only 3 microns thick—yet they are surrounded by the hardest bone in the body. This makes studies of the human cochlea so difficult. It is the most challenging tissue to study in the entire body and explains why so little is known in humans!
The cilia, often known as hair cells, are around 150–200 nanometer (1 nanometer = 1 one-millionth of a millimeter) in diameter. One can compare it with the diameter of a human hair; that is 30–100 microns (one thousand nanometers = one micron), making hair cells less than a 1/100th the diameter of a human hair.
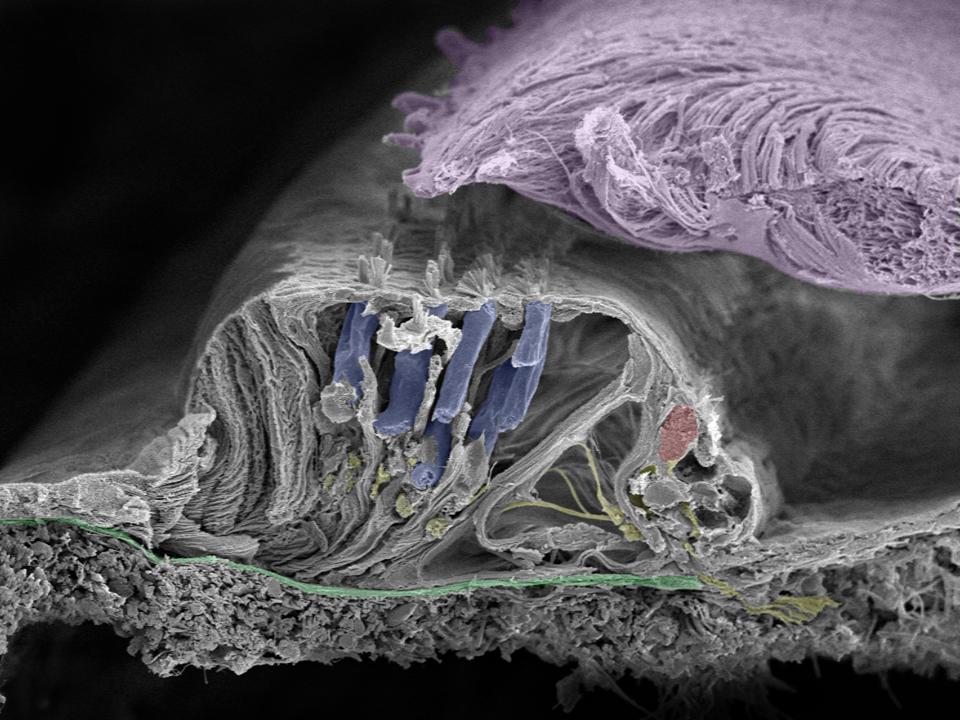 This image shows the natural beauty of the human organ of hearing—the organ of corti. The green membrane is called the basilar membrane which helps to filter the acoustic frequencies. The cilia project from the surface of the inner (red) and outer (blue) hair cells to the tectorial membrane (lilac). Nerve fibers are yellow. These structures are so precise that a movement equal to the diameter of a hydrogen atom is enough to trigger a neural response. Image taken in Innsbruck, Austria with a Zeiss field emission microscope by Prof. Rask-Andersen together with Annelies Schrott-Fischer, Rudolph Glueckert, and Kristian Pfaller.
This image shows the natural beauty of the human organ of hearing—the organ of corti. The green membrane is called the basilar membrane which helps to filter the acoustic frequencies. The cilia project from the surface of the inner (red) and outer (blue) hair cells to the tectorial membrane (lilac). Nerve fibers are yellow. These structures are so precise that a movement equal to the diameter of a hydrogen atom is enough to trigger a neural response. Image taken in Innsbruck, Austria with a Zeiss field emission microscope by Prof. Rask-Andersen together with Annelies Schrott-Fischer, Rudolph Glueckert, and Kristian Pfaller.
At hearing threshold, the shearing forces bend the hair cells less than a nanometer—which is the diameter of a hydrogen atom. Even more intriguing is that we only possess around 3,400 inner hair cells, which are the principal message-conducting cells to the brain; compare that to millions of photoreceptors in the eye. All of your inner hair cells can occupy the tip of a pin, yet they’re responsible for every single sound you hear—around 100,000 different sound modalities. With so few receptors it makes the ear especially vulnerable.
Stria Vascularis: Power Plant of the Cochlea
Our recent findings provide fascinating insight into the molecular structure of the so-called “electric power plant” or stria vascularis located in the lateral wall of the cochlea. It is responsible for the energy production and ion concentration in endolymph and essential for hair cell function.
The lateral wall is called “spiral ligament” (ligamentum spirale). This is wrong! It is not like a ligament, like in the knee, but an amazing architecture of cells that serves like an electric power plant or telephone battery—functioning with potassium ions instead of lithium. Another difference is that it does not need re-charging! All cells have different functions and are extremely well supplied with arterial blood. It is extremely vulnerable and at risk in inner ear surgery.
If the cochlear structures are damaged, the dendrites or nerve fibers connecting hair cells with the cell bodies will likely disappear. Fortunately, this degenerative process seems to stop at the level of the cell bodies but there is probably some degeneration of the cell bodies, at least with time. This cell damage induced by potassium intoxication has little healing capacity.
 Immunehistochemistry of the lateral wall of the human cochlea. The different proteins involved in the generation of electricity (know as the endocochlear potential or EP) important for hair cell function are shown in colors. The tissue produces high concentrations of potassium ions (K+) used by the hair cells. The green, red and yellow represent different molecular arrangements of ion transporters. They can also be found in the kidney. Like a car battery, the elements are surrounded by an insulator; here composed of the protein Claudin-11 (green in upper graphic picture). (Liu et al. 2017)
Immunehistochemistry of the lateral wall of the human cochlea. The different proteins involved in the generation of electricity (know as the endocochlear potential or EP) important for hair cell function are shown in colors. The tissue produces high concentrations of potassium ions (K+) used by the hair cells. The green, red and yellow represent different molecular arrangements of ion transporters. They can also be found in the kidney. Like a car battery, the elements are surrounded by an insulator; here composed of the protein Claudin-11 (green in upper graphic picture). (Liu et al. 2017)
The stria vascularis is partly destroyed in the base during conventional cochleostomies, because drilling damages the inner ear tissues. Bone dust enters the cochlea, and inflammation and fibrosis develop more easily. Today, I think it is valuable to use a technique where drilling of the bone is avoided and the inner surface of the cochlea (endosteum) is not traumatized. However, I must confess that I also sometimes make a cochleostomy, when finding the round window is challenging due to cochlear rotations. Nonetheless, I think it is important to realize the degree of trauma it infers in connection with hearing preservation surgery.
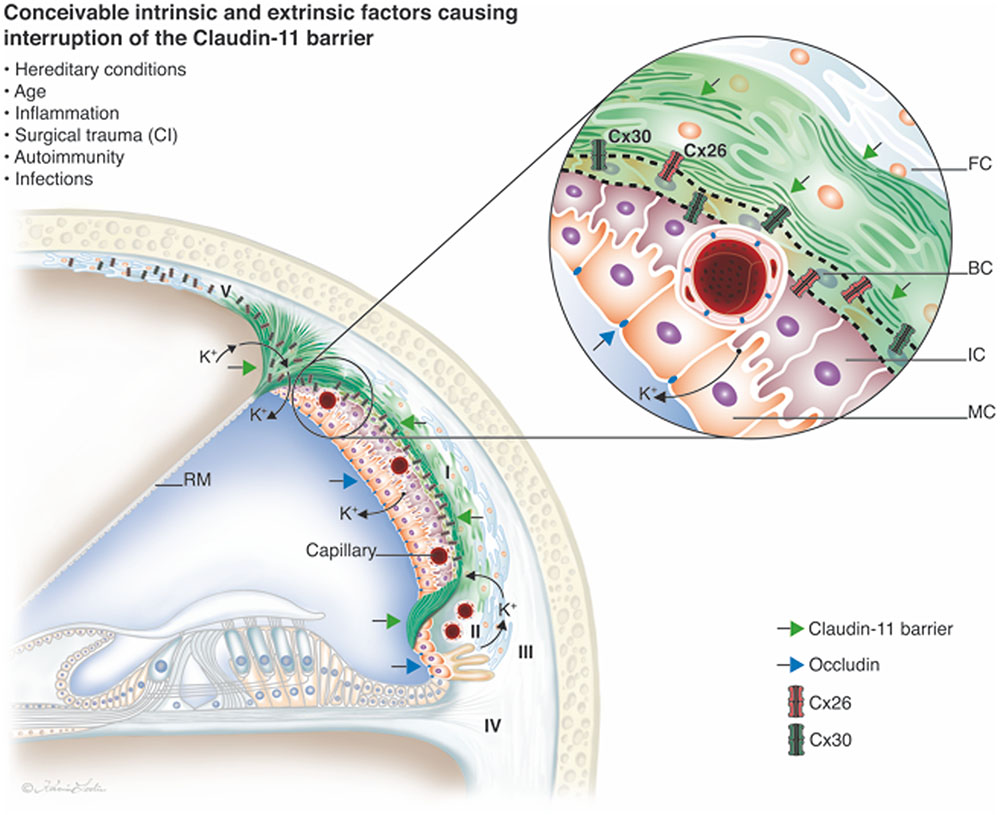 The beautiful “cochlear battery” in the lateral wall of the human cochlea. It is surrounded by an insulator; a protein called Claudin-11. The connexin proteins are abundant here and are of two types (mutated in most cased of congenital deafness). They likely help to transport and recirculate the potassium ions. (Liu et al. 2017)
The beautiful “cochlear battery” in the lateral wall of the human cochlea. It is surrounded by an insulator; a protein called Claudin-11. The connexin proteins are abundant here and are of two types (mutated in most cased of congenital deafness). They likely help to transport and recirculate the potassium ions. (Liu et al. 2017)
Staying in the Scala Tympani
It is also important that electrodes do not perforate the structures of the cochlea, especially near the first turn. If the electrode deviates into the scala vestibule, it usually penetrates the scala media, which contains the hair cells. A macrofistula develops and potassium ions will leak out and hair cells will stop working. Potassium ions also leak around the hair cell bodies and nerve cells which is toxic for them and they will degenerate near the perforation. Several reports suggest that cochlear implant results are better when electrode does not deviate into the scala vestibuli.
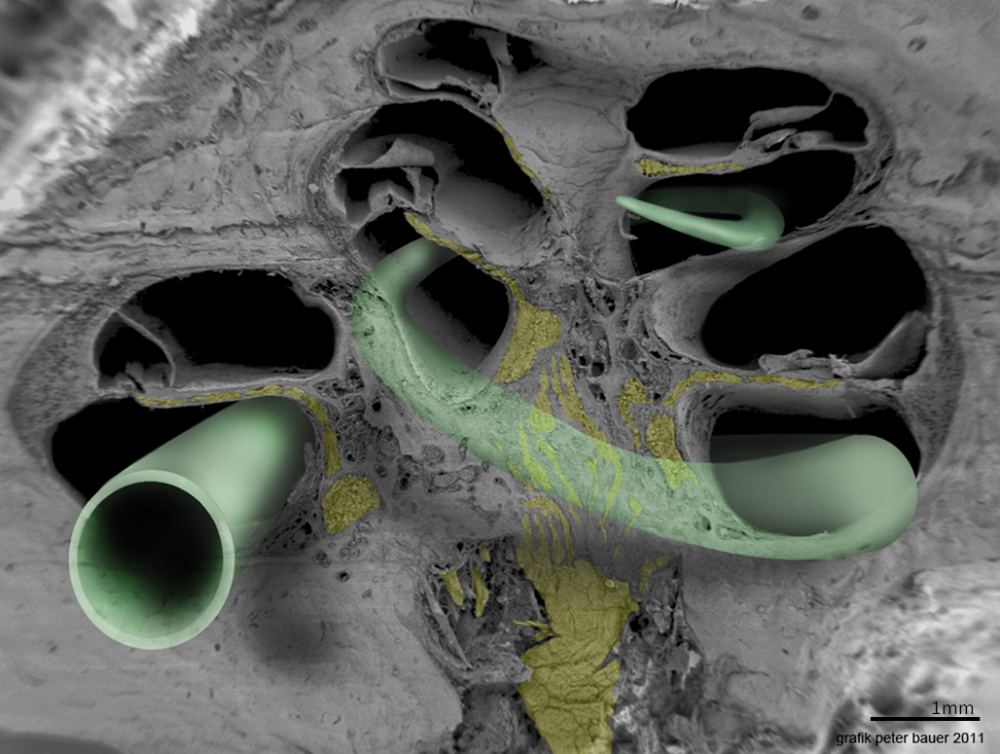 This image shows the proper positioning of a cochlear implant electrode array in the scala tympani. This picture was taken in Innsbruck, Austria with a Zeiss field emission microscope by Prof. Rask-Andersen together with Annelies Schrott-Fischer, Rudolph Glueckert and Kristian Pfaller, with rending by Peter Bauer.
This image shows the proper positioning of a cochlear implant electrode array in the scala tympani. This picture was taken in Innsbruck, Austria with a Zeiss field emission microscope by Prof. Rask-Andersen together with Annelies Schrott-Fischer, Rudolph Glueckert and Kristian Pfaller, with rending by Peter Bauer.
The modiolus is also vulnerable and the surface wall (where the CI electrode is located), faces the nerve cell bodies which can easily be damaged. The fluid in the cochlea also mix with the fluid surrounding the ganglion cells, meaning that a toxic inflammation in the cochlea will spread to the nerve.
From everything said, it seems pertinent to say that structural preservation in cochlear implantation surgery should be motivated for every patient, even if there is no residual hearing.
Thank you Prof. Rask-Andersen, we appreciate your passionate research and insightful contribution.
In part two of this series, Prof. Rask-Andersen will show us what future research may offer in hearing loss solutions—including stem cells, gene therapy, and utilizing natural immune responses to our advantage.
Learn More: Structure Preservation
Ready to learn more about how the structures inside the cochlea can be damaged? Check out this helpful guide to intracochlear fluids and the importance of the endocochlear potential.
What factors affect structure preservation outcomes? Learn how electrode array design and surgical approach can both significantly affect structure preservation and hearing outcomes.
*Not all products, features, and indications shown are available in all areas. Please contact your local MED-EL representative for more information.
Images courtesy of Prof. Rask-Andersen & team. Portrait courtesy of the Uppsala Academic Hospital.
Lie, W., Schrott-Fischer, A., Glueckert, R., Benav, H., & Rask-Andersen, H. (2017) The Human “Cochlear Battery” – Claudin-11 Barrier and Ion Transport Proteins in the Lateral Wall of the Cochlea. Front. Mol. Neurosci. 10:239.
MED-EL
Was this article helpful?
Thanks for your feedback.
Sign up for newsletter below for more.
Thanks for your feedback.
Please leave your message below.
CTA Form Success Message
Send us a message
Field is required
John Doe
Field is required
name@mail.com
Field is required
What do you think?
The content on this website is for general informational purposes only and should not be taken as medical advice. Please contact your doctor or hearing specialist to learn what type of hearing solution is suitable for your specific needs. Not all products, features, or indications shown are approved in all countries.
MED-EL



