MED-EL
Published Sep 13, 2017
EAS Candidacy for High-Frequency Hearing Loss

High-frequency hearing loss can be particularly challenging to treat. These patients have minimal hearing loss in the low frequencies (125–1000 Hz), so they are not traditional cochlear implant candidates. However, with severe-to-profound high-frequency hearing loss, hearing aids often do not provide enough benefit. Even with the use of frequency compression or frequency transposition technology in hearing aids, speech understanding can still be very difficult.1,2
That’s why MED-EL created Electric Acoustic Stimulation (EAS) systems to address the specific challenges of high-frequency hearing loss. An EAS system is a two-part approach that combines:
- Acoustic amplification for the low frequencies
- Electrical cochlear implant stimulation for the high frequencies
For patients with minimal low-frequency hearing loss and severe-to-profound high-frequency hearing loss, EAS can restore a wide frequency range for optimal hearing outcomes.2,3,4,5,6,7
This week, we’re looking at how EAS can benefit your patients—then we’ll introduce a new expanded indication for MED-EL EAS systems.
EAS for High-Frequency Hearing Loss
First, let’s start by going through a quick overview of how an EAS system works. Our latest EAS system uses the SYNCHRONY cochlear implant and the SONNET EAS audio processor.
Can’t See This Video?
Having problems viewing this video? Watch it on YouTube.
For the cochlear implant, an ultra-flexible electrode array should be gently inserted using hearing preservation surgery techniques to better protect delicate cochlear structures and preserve residual hearing. We generally recommend using the FLEX 24 or FLEX 20 electrode array.2
For acoustic amplification, the SONNET EAS audio processor sends the amplified acoustic signal through the earmold into the ear canal. As our third generation EAS processor, SONNET EAS offers:
- Directional microphones
- 6-channel acoustic amplification
- Gain of up to 48 dB
- Maximum power output of 118 dB SPL
The timing of both the electric and acoustic signals are matched, which enables a full, rich perception of sound.
Benefits of EAS
Why should you consider EAS over a high-end hearing aid for your patients? Compared to hearing aids alone, EAS offers significant benefits for recipients with high-frequency hearing loss.2,3,4,5,6,7,8
- Significantly better speech understanding
- Better hearing in noise
- Greater satisfaction in hearing
- Reduced communication effort
- More natural perception of low frequencies
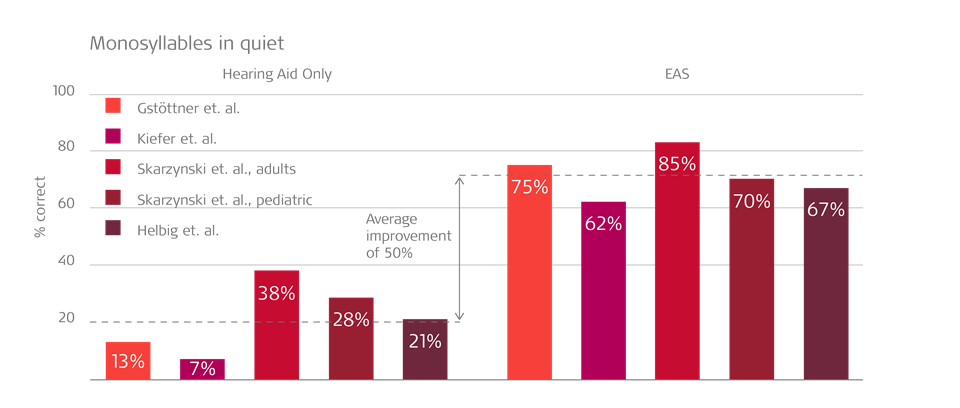 An overview of hearing aid vs EAS hearing outcomes across several studies shows consist benefits.3,4,5,6,7
An overview of hearing aid vs EAS hearing outcomes across several studies shows consist benefits.3,4,5,6,7
Candidacy for EAS
How can you determine if a patient is a candidate for EAS? Exact EAS candidacy parameters can vary by country, but these are intended to be used for general guidance.
Indication of sensorineural high-frequency hearing loss with hearing thresholds that fall within the shaded area in the charts below.
- No malformations or obstructions of the cochlea, or external ear contraindications to using hearing aid in the ear canal
- Stable air conduction thresholds
- No air-bone gap >15 dB
- Monosyllable score ≤60% at 65 dB SPL in best aided condition
- Adequate motivation and realistic expectations
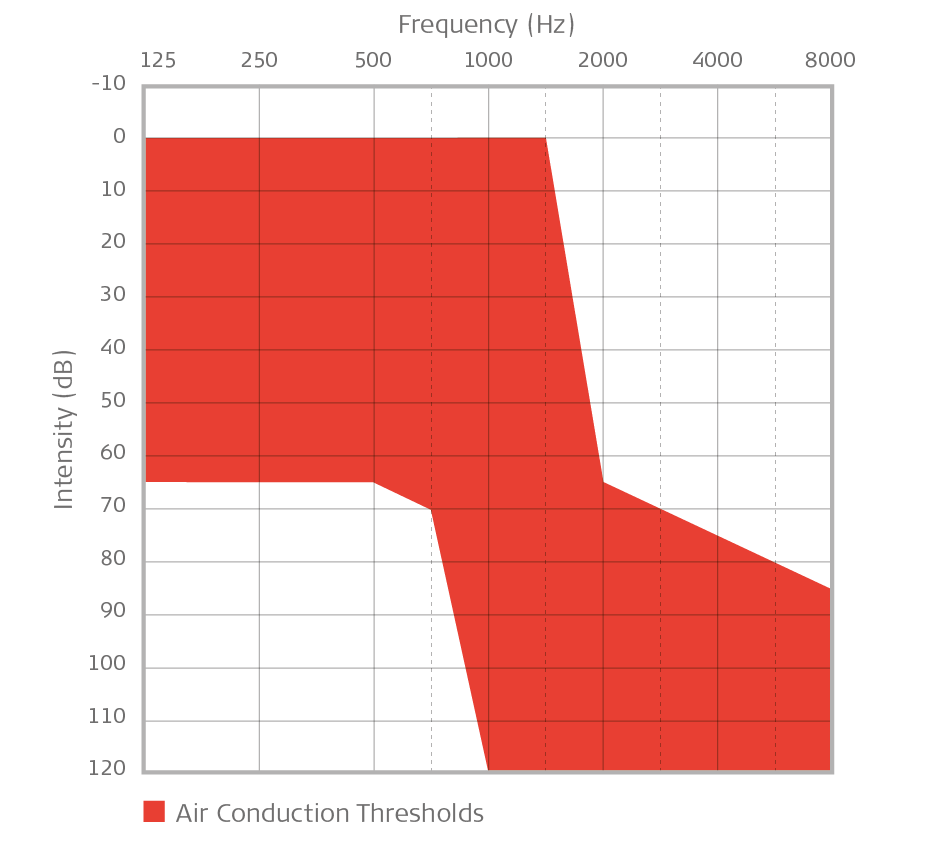 General indication for EAS: Minimal-to-moderate hearing loss up to 750 Hz, sloping down to severe-to-profound sensorineural hearing loss in the higher frequencies. Audiogram should chart within shaded area.
General indication for EAS: Minimal-to-moderate hearing loss up to 750 Hz, sloping down to severe-to-profound sensorineural hearing loss in the higher frequencies. Audiogram should chart within shaded area.
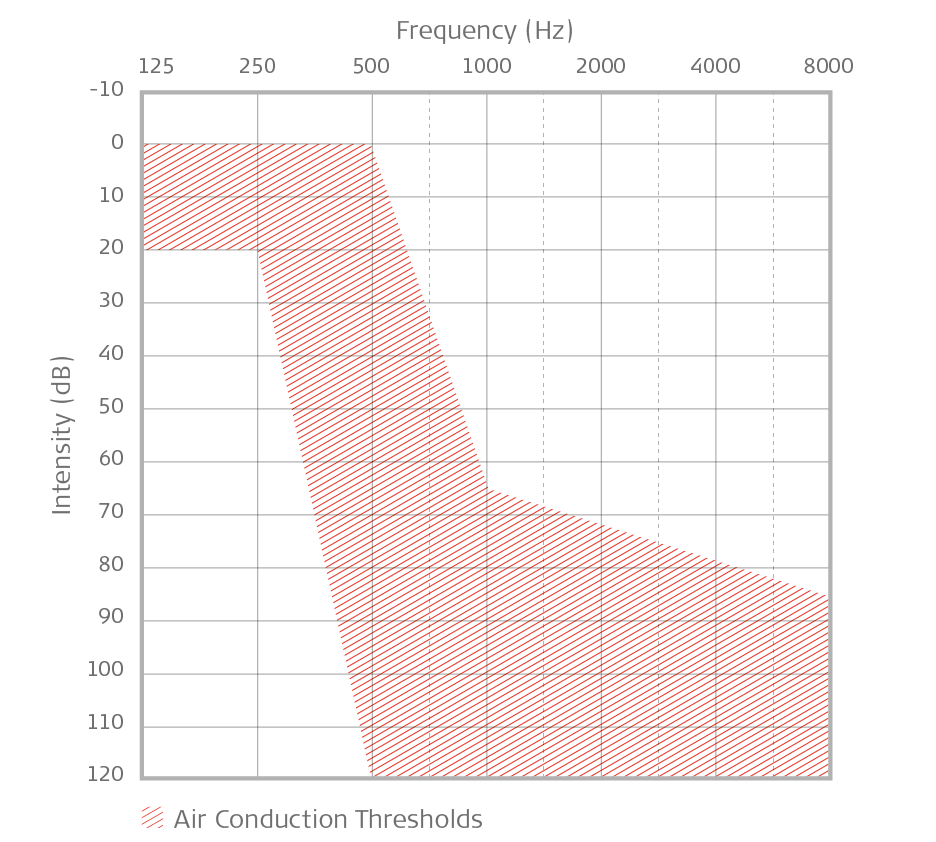
Expanded indication for EAS: No hearing loss up to 250 Hz; at 500 Hz & higher, profound hearing loss. Audiogram should chart within shaded area.
This expanded indication for EAS allows recipients to benefit from their natural residual hearing, even if they have profound hearing loss at 500 Hz and higher on audiometric frequencies.
Preserving Residual Hearing
Of course, natural hearing function is essential for EAS. Yet, every cochlear implantation procedure carries a risk to the delicate cochlear structures and residual hearing.
That’s why MED-EL electrode arrays are designed for superior atraumaticity to protect residual hearing. By combining ultra-flexible lateral wall electrode arrays with round window insertion and hearing preservation techniques, consistently high rates of hearing preservation can be achieved with MED-EL.2,8,9,10,11,12,13,14
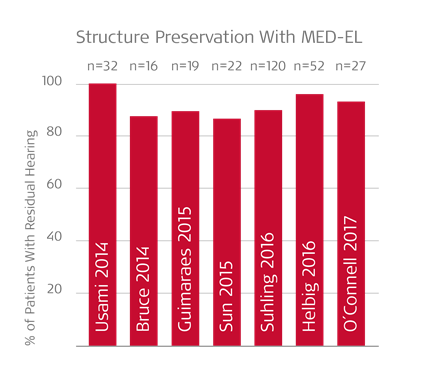
Unfortunately, other “hybrid” systems can often damage or destroy any residual hearing. In the multicenter trial of the Cochlear Nucleus Hybrid L24, 44% of patients completely lost their residual hearing—and a full 10% of patients had to later be reimplanted with a conventional cochlear implant.15
In contrast, in a recent multicenter study of MED-EL EAS systems, 97% of MED-EL recipients were able to use the acoustic component and benefit from EAS.16
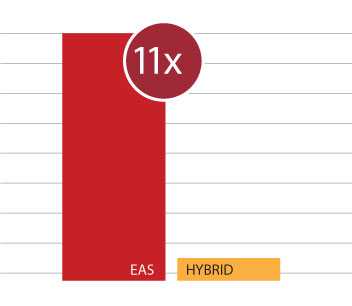
In these studies, MED-EL EAS recipients were 11 times more likely to use their acoustic component after surgery.15,16
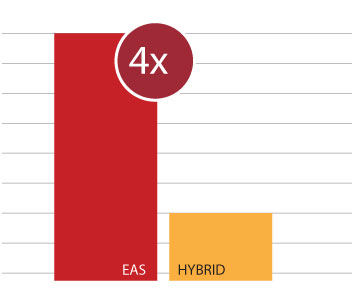
With MED-EL, EAS recipients were 4 times more likely to maintain their residual hearing after surgery.15,16
As you can see, EAS can restore the ability to hear across a wide frequency range, making it an ideal solution for high-frequency hearing loss. The benefits for your patients are significant, including:
- Better speech understanding in quiet
- Improved hearing in noise
- Greater hearing satisfaction
And as we’ve shown, MED-EL offers superior outcomes for patients who want to make the most of their natural residual hearing.2,8,15,16
Subscribe & Share
Have a question about EAS for your patients? Leave a comment below or contact us. We’ll have more articles on EAS and hearing preservation—make sure to subscribe now!
References
- Hopkins, K., Khanom, M., Dickinson, A., & Munro, K. (2014). Benefit from non-linear frequency compression hearing aids in a clinical setting: The effects of duration of experience and severity of high-frequency hearing loss. International Journal of Audiology. 53: 219–228
- Usami, S., Moteki, H., Tsukada, K., Miyagawa, M., Nishio, S.Y., Takumi, Y., Iwasaki, S., Kumakawa, K., Naito, Y., Takahashi, H., Kanda, Y., & Tono, T. (2014). Hearing preservation and clinical outcome of 32 consecutive electric acoustic stimulation (EAS) surgeries. Acta Otolaryngol. 134(7):717–727
- Gstoettner, W., Helbig, S., Maier, N., Kiefer, J., Radeloff, A., & Adunka, O.F. (2006). Ipsilateral electric acoustic stimulation of the auditory system: results of a long term hearing preservation. Audiol Neurootol. 11: 49–56
- Kiefer, J., Pok, M., Adunka, O., Stürzebecher, E., Baumgartner, W., Schmidt, M., Tillein, J., Ye, Q., & Gstoettner, W. (2005). Combined electric and acoustic stimulation of the auditory system: results of a clinical study. Audiol Neurootol. 10(3):134–144.
- Helbig, S., Van de Heyning, P., Kiefer, J., Baumann, U., Kleine-Punte, A., Brockmeier, H., Anderson, I., & Gstoettner, W. (2011). Combined electric acoustic stimulation with the PULSARCI100 implant system using the FLEXEAS electrode array. Acta Oto-Laryngologica. 131(6):585–595.
- Skarzynski, H., Lorens, A., Piotrowska, A., & Anderson, I. (2006). Partial deafness cochlear implantation provides benefit to a new population of individuals with hearing loss. Acta Oto-Laryngologica. 126(9):934–940.
- Skarzynski, H., Lorens, A., Piotrowska, A., & Anderson, I. (2007). Partial deafness cochlear implantation in children. Int J Pediatr Otorhinolaryngol. 71(9):1407–1413.
- Helbig, S., Adel, Y., Rader, T., Stöver, T., & Baumann, U. (2016). Long-term hearing preservation outcomes after cochlear implantation for electric-acoustic stimulation. Otol Neurotol. 37(9):353–359.
- Bruce, I.A., Felton, M., Lockley, M., Melling, C., Lloyd, S.K., Freeman, S.R., & Green, K.M. (2014). Hearing preservation cochlear implantation in adolescents. Otol Neurotol. 35(9):1552–1559.
- Guimarães, A.C., Carvalho, G.M., Duarte, A.S., Bianchini, W.A., Sarasty, A.B., Gregorio, M.F., Zernotti, M.E., Sartorato, E.L., & Castilho, A.M. (2015). Hearing preservation and cochlear implants according to inner ear approach: multicentric evaluation. Braz J Otorhinolaryngol. 81(2):190-196
- Sun, C.H., Hsu, C.J., Chen, P.R., & Wu, H.P. (2015). Residual hearing preservation after cochlear implantation via round window or cochleostomy approach. Laryngoscope. 125(7):1715–1719.
- Suhling, M.C., Majdani, O., Salcher, R., Leifholz, M., Buechner, A., Lesinski-Schiedat, A., & Lenarz, T. (2016) The impact of electrode array length on hearing preservation in cochlear implantation. Otology & Neurotology. 37(8):1006–1015.
- O’Connell, B.P., Hunter, J.B., Haynes, D.S., Holder, J.T., Dedmon, M.M., Noble, J.H., Dawant, B.M., & Wanna, G.B. (2017). Insertion depth impacts speech perception and hearing preservation for lateral wall electrodes. Laryngoscope. [epub ahead of print].
- Nordfalk, K., Rasmussen, K., Hopp, E., Bunne, M., Silvola, J.T., & Jablonski, G.E., (2016). Insertion Depth in Cochlear Implantation and Outcome in Residual Hearing and Vestibular Function. Ear Hear. 37(2):e129–137.
- Roland, J.T., Gantz, B.J., Waltzman, S.B., & Parkinson, A.J. (2016) Multicenter Clinical Trial Group.United States multicenter clinical trial of the cochlear nucleus hybrid implant system. Laryngoscope. 126(1):175–181.
- EAS Clinical Trial: IDE G040002 Final Report “Electric-Acoustic Systems Clinical Trial”
*Not all products, indications, and features shown are available in all areas. Please contact your local MED-EL representative for more information.
MED-EL
Was this article helpful?
Thanks for your feedback.
Sign up for newsletter below for more.
Thanks for your feedback.
Please leave your message below.
CTA Form Success Message
Send us a message
Field is required
John Doe
Field is required
name@mail.com
Field is required
What do you think?
The content on this website is for general informational purposes only and should not be taken as medical advice. Please contact your doctor or hearing specialist to learn what type of hearing solution is suitable for your specific needs. Not all products, features, or indications shown are approved in all countries.
MED-EL



