MED-EL
Published Sep 26, 2019
Always A Step Ahead: New BCI 602 Active Bone Conduction Implant for BONEBRIDGE
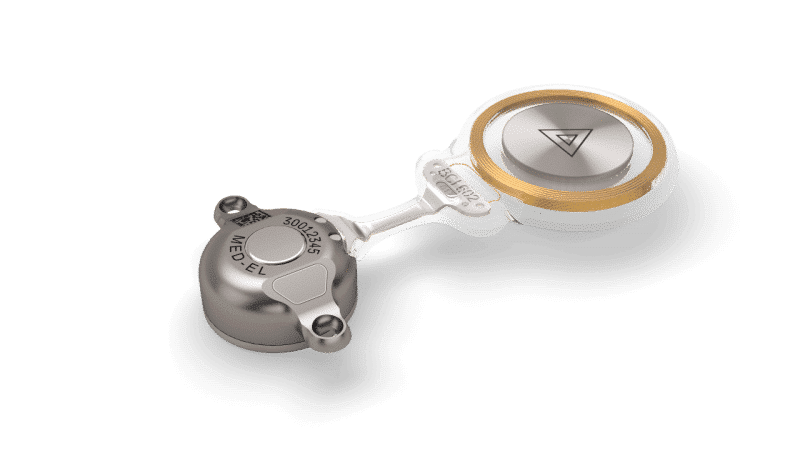
Exciting news! We just launched the next generation of our outstanding BONEBRIDGE active bone conduction implant.
In 2012, we changed the world of bone conduction with the first generation of BONEBRIDGE, the world’s first and only active bone conduction implant. Since then, we’ve been working closely with surgeons and clinicians to develop the next generation of BONEBRIDGE.
The new BCI 602 delivers the same great hearing performance as the BCI 601, but we’ve cut the drilling depth nearly in half—down to 4.5 mm. We’ve also upgraded the implant fixation with self-drilling screws to streamline the surgical process.
Great news: BONEBRIDGE BCI 602 is now also FDA cleared for the United States. BONEBRIDGE is indicated for candidates ages 12 years and older in the United States.
Can’t See This Video?
Having problems viewing this video? Watch it on YouTube.
Active Bone Conduction
BONEBRIDGE offers unique advantages, because it’s an active and transcutaneous bone conduction implant. This means the powered floating mass transducer (BC-FMT) that delivers the mechanical sound energy is built into the implant, not the audio processor.
 Why is this so important? This unique design allows direct-drive active bone conduction, while still enabling wireless transmission through healthy, intact skin. This means the SAMBA audio processor can connect with gentle magnetic fixation instead of a BAHA abutment with an open wound or tight pressure of a headband or passive magnetic BAHA systems.
Why is this so important? This unique design allows direct-drive active bone conduction, while still enabling wireless transmission through healthy, intact skin. This means the SAMBA audio processor can connect with gentle magnetic fixation instead of a BAHA abutment with an open wound or tight pressure of a headband or passive magnetic BAHA systems.
This also allows the microphones of the audio processor to be separated from the output of the transducer, so there are no issues with feedback. Other BAHA systems use a combined audio processor/transducer, which can lead to feedback issues and limit effective output.
- Direct-drive active bone conduction
- No open wound from percutaneous screw
- No microphone feedback
Drilling Depth
What’s new in BCI 602? The most significant change is that we’ve optimized the size and configuration of the BC-FMT. This upgrade enables the same power output for effective amplification, but with nearly 50% less drilling depth.
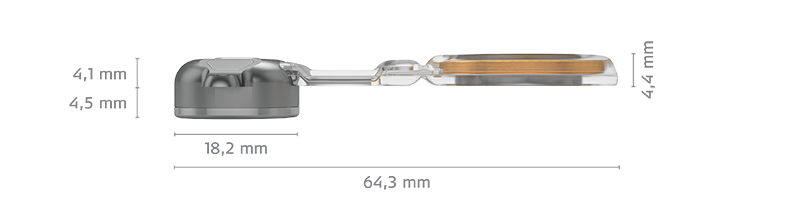
Now, you only need to drill to a depth of 4.5 mm – and only 3.5 mm if using BCI 602 Lifts (1 mm).
- 4.5 mm drilling depth (almost 50% reduction)
- Anatomically less restricting
- Faster, more straightforward drilling
Self-Drilling Screws
BONEBRIDGE is securely anchored to the bone with a pair of screws and fixation wings. For the BCI 602, we’re introducing self-drilling screws that enable fast, secure implant fixation. The single-use screwdriver included with the implant simplifies clinical logistics.
- No need for pre-drilling
- Robust two-point implant fixation
- Fast, secure placement
First Surgical Experiences: BONEBRIDGE BCI 602
Of course we’re excited about the new BONEBRIDGE BCI 602, but what do surgeons have to say about their first experiences with the new implant?
Can’t See This Video?
Having problems viewing this video? Watch it on YouTube.
Dr. Henning Frenzel
I just finished my first implantation of the BCI 602, and I have to say, my first impression is very good, I’m absolutely excited about the new design. All the work we put in the new design, all together makes a great benefit and a great step ahead for that implant.
The main benefits of the new BONEBRIDGE BCI 602 are: First, the self-drilling screws that make the handling of the implant much easier. You don’t need to pre-drill, you don’t need specialized surgical tools. The new screwdriver has good handling and the screws are very easy to use.
And the second advancement is the reduced depth of the boney bed for the BC-FMT; it’s only ~4 millimeters. The reduced drilling depth reduces the drilling time and reduces the likelihood of Dura exposure and that reduces surgical time significantly. The implantation of the new BONEBRIDGE will be much faster, and actually it has been during the first implantation from my side.
The new design with the reduced depth of implantation allows new positioning, more options, and there will be more options of positioning coming I’m sure.
Patients will benefit from the new BONEBRIDGE, in the way that more patients can receive the new implant. I’m thinking about children and young children especially, because of the reduced depth of the new BONEBRIDGE.
I’m looking really forward to the next surgery: We have three more implantations and I’m really happy and really excited about it.
Prof. Dr. Georg Mathias Sprinzl
The new BONEBRIDGE is a masterpiece of Austrian engineering and we are happy to have this implant to serve our patients. The implantation of the new BONEBRIDGE is a safe and fast and very quick approach. So we have an implant in our hands which is really very joyful for the surgeon, because surgery is very, very easy.
We have to remove just a little piece of bone of the skull and then can easily adjust the FMT to the skull and fix the FMT with two screws, which are self-tapping screws, so it’s an easy and straightforward approach. It’s really a pleasure to work with that new implant.
The main benefit for me as a surgeon is that I could reduce my operative time. We did now the first surgery, and the first surgery time was about 15 minutes from the incision of the skin to the closure of the skin, and this reflects I think the easiness of the surgery itself and also the safety of the surgery.
It makes the surgery more safe, because I don’t have to expose the dura, I don’t have to expose the sigmoid sinus, and the implantation is really very, very easy, and I think that in the long run this kind of surgery can also be done by less experienced surgeons.
The fixation is very easy. We have self-tapping screws and the screws can be screwed very easily into the skull, so you don’t need to have to pre-drill; it’s quite easy, that’s it.
So you don’t have to deal with dura, you don’t have to deal with sigmoid sinus, and to my knowledge this implant at the moment is the best implant in the world—in my opinion, the new BONEBRIDGE from MED-EL is the top bone conduction implant.
The main benefit for my patients is that they have less trauma in the skull; they have a very fast and quick recovery from the surgery; and surgery itself, it’s made easier, and so this is the best benefit. The best argument for this implant is less trauma to the patient, quick activation, and very good results.
Dr. Astrid Magele
I just finished my first BONEBRIDGE surgery of the new generation, and I am very happy that it went very well. I am very happy that I have been involved right from the start in a team of the BONEBRIDGE implantations, and now I see that it is improved very well, and I think this is because we did a lot of work to improve the surgery, to improve the implant, and to get better conditions for the patients.
The surgery now was very easy, because we have a smaller implant, especially the BC-FMT was smaller and so also the surgery duration decreased. And also the self-cutting screws are very easy to handle, so you do not need to use a drill like before. For me as a surgeon, it’s very easy to handle.
What I like most about this implant is that it is a transcutaneous system, so the skin is fully closed over the implant and I think this is a big advantage for the patients. For the patient it’s also an advantage that you can reduce the surgery time, and you have a wide spectrum for indication, so many patients will get this BONEBRIDGE.
Dr. Thomas Rasse
An important advantage of the new BONEBRIDGE is that we have a smaller implant bed. The thickness of the implant is reduced to 4.4 millimeters. The fixation of the implant is now perfect because of the self-drilling screws.
Right after a surgery now the first impressions are great. It’s fantastic to see how the company uses the hints and the feedback of the surgeons to improve the implant. And so this is like symbiotic activity between the developer and the ENT surgeon and that’s fantastic.
Right now after surgery we can say that the surgical time is reduced a lot, because of the screws and because of the reduced thickness of the implant. The most benefit for me as a surgeon is that I don’t have to explore the sinus or the dura and therefore I can be much faster.
The main benefit for the patient is that you can implant the device in narrow anatomical situations as in kids or if there had been several surgeries before.
Prof. Dr. Javier Gavilán
My first implantation with the new BONEBRIDGE has been a success. I’ve been very happy to see the improvements of the device and I think we’ve made great step forward with this.
I think the new BONEBRIDGE is a clear innovation when you compare it to the previous one. It’s smaller, it’s thinner, and it’s easy to fixate to the bone. And those three things in my opinion are really important.
The new BONEBRIDGE is obviously shorter in operative time. You don’t need to drill as much as for the previous one—it’s almost half of the depth of the previous one. And you don’t need to prepare the holes, and drill the holes, and then put the screws.
So this one is shorter, both in bone-depth drilling and fixation of the implant. With the new one, I hope we can shorten the duration of the operation by 20 minutes. With less drilling, less time doing the holes for the fixation, I think you could save around 15 to 20 minutes
As a surgeon, for me the new device has two main advantages. One is obviously it’s thinner, so you have to drill less; you have less risk to expose the dura or the sigmoid sinus.
And the main advantage of this new device, for me, is something I’ve been fighting for during the last years since the first BONEBRIDGE came to market, and it’s the use of self-drilling screws. I love self-drilling screws.
When the old device was presented seven years ago, I wondered why we were not using self-drilling screws. They have been used for many other purposes in medicine and surgery and they needed to be used also here, so I am glad that MED-EL accepted these new screws for this device. It took seven years, but we got it. We now have self-drilling screws which are faster, and in my opinion, safer.
As a surgeon, I consider that the feedback of surgeons for developing new devices is crucial, because we are the ones working with the device on a daily basis and we know where we have difficulties. We know how to change, what we want to change, in these devices.
I’m glad that this time we were heard, and our opinions counted for the new device. MED-EL asked us this time how to change, how to improve the BONEBRIDGE, which is a great device.
Subscribe & Share
Ready to learn about getting BONEBRIDGE BCI 602 for your clinic? Contact your local MED-EL team for more details on local availability!
BONEBRIDGE is made for easyMRI, enabling straightforward 1.5 Tesla MRI scans. Hear more from Prof. Dr. Gavilán about MRI and BONEBRIDGE here.
Check out this guide to candidacy for our bone conduction solutions.
Subscribe to make sure you get all the latest articles from the MED-EL Professionals Blog.
*Not all products, indications, and features shown are available in all areas. Please contact your local MED-EL representative for more information.
**Recipients with BONEBRIDGE BCI 602 may be safely MRI scanned at 1.5 Tesla following the conditions detailed in the instructions for use.
MED-EL
Was this article helpful?
Thanks for your feedback.
Sign up for newsletter below for more.
Thanks for your feedback.
Please leave your message below.
CTA Form Success Message
Send us a message
Field is required
John Doe
Field is required
name@mail.com
Field is required
What do you think?
The content on this website is for general informational purposes only and should not be taken as medical advice. Please contact your doctor or hearing specialist to learn what type of hearing solution is suitable for your specific needs. Not all products, features, or indications shown are approved in all countries.
MED-EL



