MED-EL
Published Jun 13, 2018
Bone Conduction Solutions for Single-Sided Deafness: ADHEAR & BONEBRIDGE
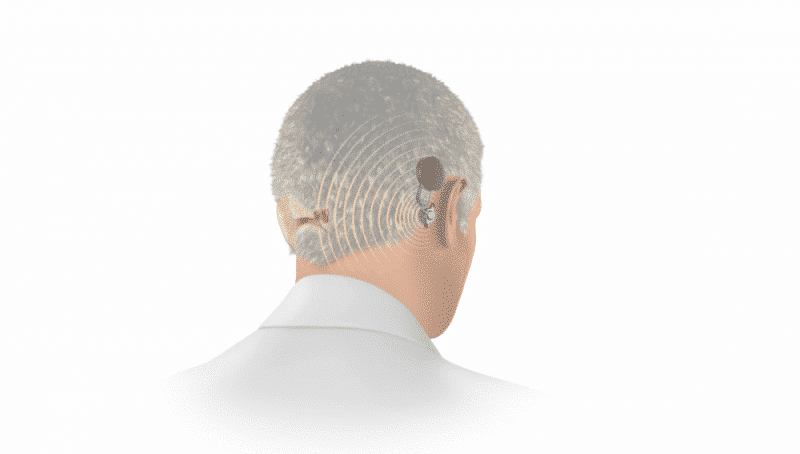
Hearing loss that only affects one ear is known as unilateral hearing loss. Unilateral hearing loss has been estimated to affect approximately 10% of the general population. And when unilateral hearing loss is severe-to-profound, it’s often referred to as single-sided deafness (SSD).1
Earlier, we looked at how a cochlear implant can be an effective treatment option for sensorineural single-sided deafness.
However, a cochlear implant may not be the right treatment option for a number of patients with single-sided deafness. By design, a cochlear implant requires a functional auditory nerve on the deafened side.
Usami et al. (2017) found that up to 40% of cases of single-sided deafness in children were caused by cochlear nerve deficiency. In these cases of auditory nerve hypoplasia or aplasia, or other retrocochlear hearing loss, a cochlear implant may not provide sufficient benefit.1
Thankfully, there’s an effective treatment option for these candidates—a bone conduction hearing system. A bone conduction system for SSD sends sounds from the deafened side to the hearing cochlea.
Today, we’re going to look at our two bone conduction hearing systems. We’ll see how BONEBRIDGE and ADHEAR can both be excellent treatment options for single-sided deafness.
How Bone Conduction Works in Single-Sided Deafness
Let’s start with the basics of bone conduction, and how BONEBRIDGE and ADHEAR can be effective options for patients with single-sided deafness.
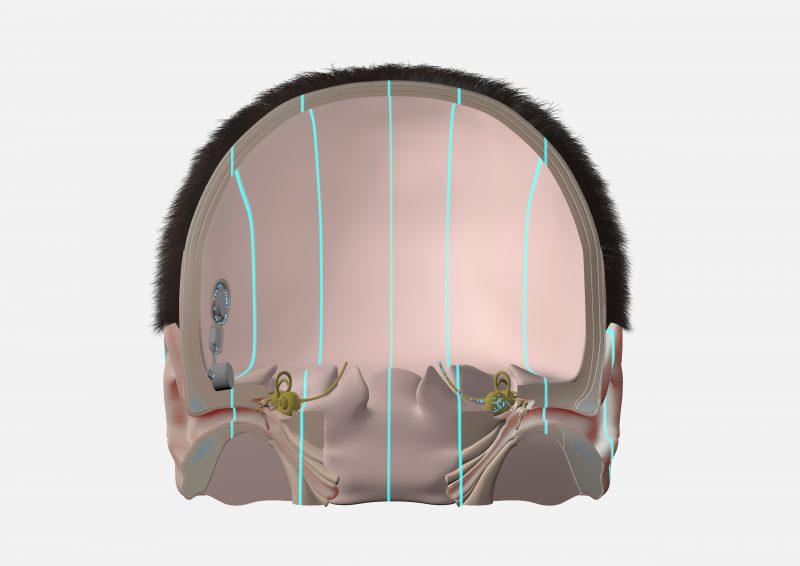
A bone conduction system uses mechanical vibrations to send sounds from the deafened side to the hearing cochlea on the other side of the head.
With BONEBRIDGE, the active bone conduction implant is placed completely under the skin on the deafened side. The SAMBA audio processor detects sounds from this side, and transmits the sounds as electronic signal to the implant. The BONEBRIDGE implant converts these electronic signals into mechanical vibrations that travel directly through the bone.
 The BONEBRIDGE Active Bone Conduction implant offers direct sound transmission for optimal hearing outcomes.
The BONEBRIDGE Active Bone Conduction implant offers direct sound transmission for optimal hearing outcomes.
With ADHEAR, the audio processor is attached to the skin using a simple adhesive adapter. This makes ADHEAR a non-surgical option. The ADHEAR audio processor transmits the vibrations through the skin to the bones of the head.

The ADHEAR audio processor and adhesive adapter offers a unique non-surgical treatment option for SSD.
When used to treat single-sided deafness, the mechanical sound vibrations of BONEBRIDGE and ADHEAR travel across the bones of the head from the deafened side to the hearing cochlea. This offers a natural sound quality with only one hearing cochlea, and effectively eliminates the head shadow effect.
Head shadow effect: The head is a natural barrier between the ears that muffles higher frequencies (1000 Hz and up) by as much as 20 dB when sounds arriving from the opposite side travel around the head to reach the ear. Without an implant, sounds on the deafened side are very difficult to hear.
With BONEBRIDGE or ADHEAR, your patients can hear sounds coming from any direction. This offers many benefits, including:2,3,4,5
- Improved speech understanding
- Easier listening in challenging environments
- No head shadow effect
- Improved quality of life
BONEBRIDGE & ADHEAR Candidacy for Single-Sided Deafness
Who is a candidate for BONEBRIDGE or ADHEAR? The following guidelines can help you better understand candidacy guidelines for BONEBRIDGE and ADHEAR for your patients with single-sided deafness.
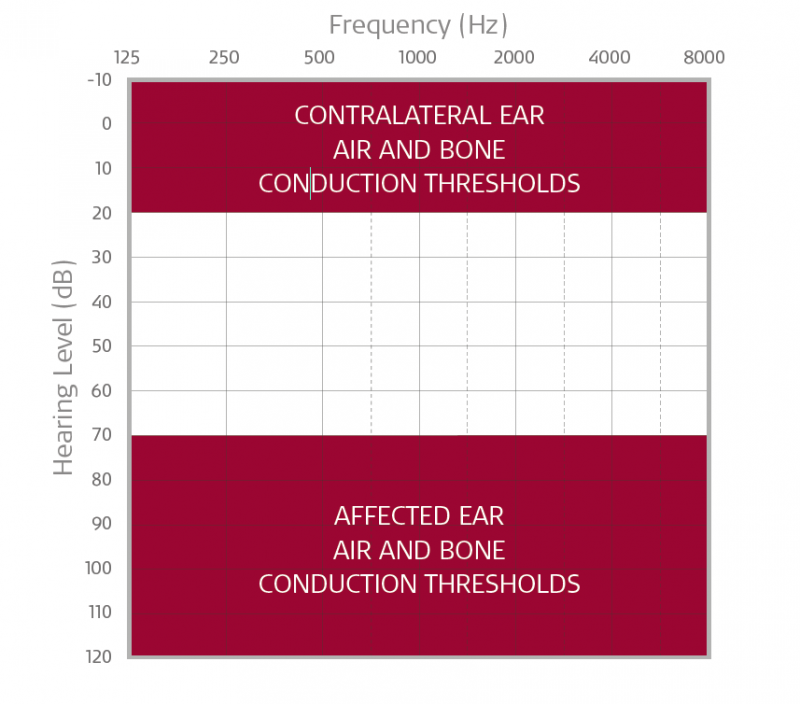 BONEBRIDGE or ADHEAR may be an ideal treatment option if your patient has severe-to-profound sensorineural hearing loss in the affected ear with hearing thresholds that fall within the shaded area in the chart above. Please note, candidacy is 90+ dB (profound SNHL) for candidates in the United States.
BONEBRIDGE or ADHEAR may be an ideal treatment option if your patient has severe-to-profound sensorineural hearing loss in the affected ear with hearing thresholds that fall within the shaded area in the chart above. Please note, candidacy is 90+ dB (profound SNHL) for candidates in the United States.
- Indication of normal hearing in the contralateral ear with hearing thresholds that fall within the shaded area in the chart above
- Air & bone conduction thresholds in the contralateral ear should be 20 dB or less.
- Absence of retrocochlear and central auditory disorders in the contralateral ear
- Adequate motivation and expectations
- Age 5 years or older for BONEBRIDGE (12 years or older for candidates in the United States)
- Anatomy that allows appropriate placement of the implant as determined by CT scan (BONEBRIDGE only)
When should you choose an ADHEAR, and when is a BONEBRIDGE a better option?
ADHEAR is a non-surgical hearing system, so you can easily try it out to see if it is a good option for each patient. This lets your patient immediately and realistically experience the sound quality of ADHEAR.
And if your patient needs greater amplification, BONEBRIDGE has the advantage of direct sound transmission and higher dynamic range for optimal hearing outcomes.
BONEBRIDGE & ADHEAR vs. CROS Hearing Aids
What sets our BONEBRIDGE and ADHEAR systems apart from a CROS hearing aid system?
Contralateral routing of signals (CROS) hearing aids are a common first step treatment option for SSD. However, with a CROS hearing aid, your patients would need to wear a hearing aid transmitter or receiver in both ears.
CROS hearing aids can have several disadvantages:2
- Uncomfortable to wear
- Occlusion effect can reduce hearing in good ear
- As low as 10% uptake/acceptance rate
In contrast, both BONEBRIDGE and ADHEAR are exceptionally comfortable and are worn completely off the ear. With BONEBRIDGE, the compact SAMBA audio processor easily disappears under hair. And there’s no occlusion effect, so natural hearing is not affected.
BONEBRIDGE & ADHEAR vs. Bone Anchored Hearing Aids
There are other bone conduction systems on the market, including the BAHA™ and PONTO™ systems. These systems generally use a bone-anchored percutaneous abutment or passive magnetic implant. These bone anchored hearing aids have significant drawbacks, including:
- High rate of infection & skin complications
- Permanent open wound
- Risk of osseointegration failure
- High audio processor profile
- Limited wearing comfort
That’s why BONEBRIDGE and ADHEAR both offer clear advantages for candidates with SSD. As the only active bone conduction implant, BONEBRIDGE allows for healthy skin between the audio processor and implant. And as the only non-surgical option without uncomfortable pressure, ADHEAR also offers healthy skin and simple access to immediate hearing benefit.
- Optimal sound transmission
- High wearing comfort
- Low profile audio processor design
- Healthy skin
- Immediate hearing benefit
- Natural sound quality
So, as you can see, both the BONEBRIDGE and ADHEAR bone conduction systems can offer many benefits for your patients with single-sided deafness.
Get a first-person view of procedure for implanting BONEBRIDGE: Check out this BONEBRIDGE surgical case study with Prof. Dr. Joachim Müller.
*Not all products, indications, and features shown are available in all areas. Please contact your local MED-EL representative for more information.
References
- Usami, SI., Kitoh, R., Moteki, H., Nishio, S.Y., Kitano, T., Kobayashi, M., Shinagawa, J., Yokota, Y., Sugiyama, K., & Watanabe, K. (2017) Etiology of single-sided deafness and asymmetrical hearing loss. Acta Otolaryngol. 137(565):S2-S7.
- Wendrich, A.W., Kroese, T.E., Peters, J.P.M., Cataani, G., & Grolman, W. (2017). Systematic review on the trial period for bone conduction devices in single-sided deafness: Rates and reasons for rejection. Otol Neurotol. 38(5):632-641.
- Sprinzl, G.M., & Wolf-Magele, A. (2016) The Bonebridge Bone Conduction Hearing Implant: indication criteria, surgery and a systematic review of the literature. Clin Otolaryngol. 41(2):131-43.
- Laske, R.D., Röösli, C., Pfiffner, F., Veraguth, D., & Huber, A.M. (2015) Functional results and subjective benefit of a transcutaneous bone conduction device in patients with single-sided deafness. Otol Neurotol. 36(7):1151-6
- Salcher, R., Zimmermann, D., Giere, T., Lenarz, T., & Maier, H., (2017) Audiological results in SSD with an active transcutaneous bone conduction implant at a retrosigmoidal position. Otol Neurotol. 38(5):642-647.
MED-EL
Was this article helpful?
Thanks for your feedback.
Sign up for newsletter below for more.
Thanks for your feedback.
Please leave your message below.
CTA Form Success Message
Send us a message
Field is required
John Doe
Field is required
name@mail.com
Field is required
What do you think?
The content on this website is for general informational purposes only and should not be taken as medical advice. Please contact your doctor or hearing specialist to learn what type of hearing solution is suitable for your specific needs. Not all products, features, or indications shown are approved in all countries.
Paul Radomski
September 21, 2023
I have a cochclear Baha 5 power and looking for options. how is yours different and compare to the baha 6
MED-EL
September 21, 2023
Hi Paul, thanks for your comment. We recommend getting in touch with your local MED-EL team via https://www.medel.com/clinic-finder as they are best equipped to help you on-site. Kind regards, Gordana
MED-EL




Conversation
1 Comment