MED-EL
Published Apr 18, 2018
Expanded Candidacy: Auditory Brainstem Implants
For many years, cochlear implants have been a very effective treatment for sensorineural hearing loss. Even patients with profound sensorineural hearing loss can have excellent hearing outcomes. Cochlear implants are designed to bypass non-functioning hair cells in the cochlea, allowing stimulation to reach the auditory nerve and travel to the auditory center of the brain.
But what can you do for a patient who doesn’t have a fully functioning auditory nerve? What if your patient has auditory nerve hypoplasia, severely ossified cochleae, or completely absent auditory nerves?
This is known as neural hearing loss or retrocochlear hearing loss. Thankfully, there is a treatment option to provide access to sound for these candidates.
Today, we’re looking at how an auditory brainstem implant can bypass the auditory nerve to send sound signals right to the brain. Even better, it’s now approved to help a much wider range of candidates with neural hearing loss—including infants (12 months and older) and young children.1,2,3
How an Auditory Brainstem Implant Works
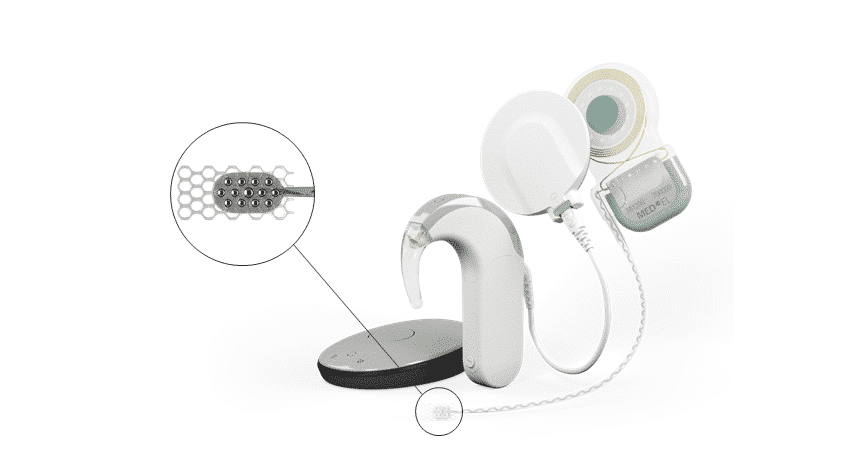
Let’s start with an overview of the system. An auditory brainstem implant (ABI) is similar in form to our cochlear implants. Like a cochlear implant system, an external audio processor detects sounds and sends coded signals to the implant. However, the ABI utilizes a much different electrode array.
The ABI electrode array is a 12-electrode paddle that is placed directly on the cochlear nucleus of the brainstem. Essentially, the cochlear nucleus is the next step in the neural pathway between the auditory nerve and brain.
 The SYNCHRONY ABI system from MED-EL can utilize any current MED-EL CI audio processor.
The SYNCHRONY ABI system from MED-EL can utilize any current MED-EL CI audio processor.
This bypasses a non-functioning auditory nerve and allows sound signals to be sent on to the brain. This makes ABI an option for candidates with non-functioning or even completely absent cochlea or auditory nerve. There is a cochlear nucleus for each auditory nerve, so bilateral auditory brainstem implants are possible.
Although the surgery is more complex, it can be considered a safe procedure for both children and adults. MED-EL auditory brainstem implants have been in use for more than 20 years.4,5

The MED-EL ABI electrode array has 12 individual electrode contacts with independent current sources.
ABI for Neurofibromatosis Type 2
Up until recently, an auditory brainstem implant was only indicated for patients with neurofibromatosis type 2 (NF2). Neurofibromatosis type 2 is a genetic disorder that is characterized by the development of non-cancerous tumors along the nervous system. These vestibular schwannomas (also known as acoustic neuromas) often form on the auditory nerve, and surgical removal of these NF2 tumors can damage the auditory nerve.1
Neurofibromatosis type 2 generally presents in adolescence or young adulthood, so candidacy was previously limited to patients age 15 years or older, with NF2 and bilateral non-functioning auditory nerves.
For individuals with NF2, it’s important to understand that hearing outcomes with an ABI for NF2 are different for each patient. Positive hearing outcomes may range from speech understanding with support from lip reading to being able to use the telephone.1,5
Expanded Indication: ABI for Non-NF2
Recently, MED-EL received CE-mark approval for much broader ABI candidacy. Now, MED-EL ABI is indicated for patients 12 months & older who cannot benefit from a cochlear implant due to non-functional auditory nerves. This means both congenital and accrued etiologies can be treated by an ABI, including:
- Auditory nerve aplasia
- Auditory nerve hypoplasia
- Head trauma
- Non-NF2 tumor
- Severe cochlear ossification
This is very important, because now candidates of essentially any age have access to a treatment for neural hearing loss. Let’s look at the possibilities of this expanded indication for auditory brainstem implants.
Auditory Brainstem Implants for Children
Research has shown promising results for ABI in young children, especially for children who had some access to hearing before implantation. It’s important to note that early intervention is a key to success when adapting to new neural input. And as with cochlear implants, neural plasticity is more reliable in young children.3,6
For children who are implanted early enough, auditory brainstem implants can offer positive auditory hearing outcomes, including open set speech recognition and ability to use telephone. Improvement takes time and dedicated rehabilitation, but significant benefits can be achieved.
This is especially important, because for children with insufficient auditory nerve function (e.g. cochlear nerve hypoplasia), an ABI can provide significantly better outcomes than a cochlear implant over time for these candidates.3,6
- Positive auditory outcomes3,6
- Improved quality of life2
- Cognitive benefits3
- Safe access to MRI7
- Possibility of mainstream education3
Auditory Brainstem Implants for Adults
This expanded indication also covers adults for both NF2 or non-NF2. Non-NF2 neural hearing loss could include accident-related trauma to the auditory nerve. Other possible causes could include bilateral cochlear ossification from otosclerosis or meningitis.4
Positive hearing outcomes are generally possible for adults, but early intervention is still important. If the auditory centers of the brain have had recent access to sound, a more robust auditory system may have developed. In turn, this may lead to greater success with an ABI.1
For adults with neural hearing loss, our expanded candidacy now offers these individuals greater opportunity to benefit from an ABI.
- Improved listening ability1,2,5
- Safe access to MRI7
- Improved quality of life2
Subscribe & Share
Want to read more about auditory brainstem implants from MED-EL? This MRI case study gives excellent insight into how the SYNCHRONY ABI system offers safe, comfortable access to MRI:
And don’t miss this article from Prof. Javier Gavilán on his method for assessing the functionality of the auditory nerve in possible ABI candidates.
Have a question about ABI for your patients? Contact your local MED-EL representative or use our simple contact form.
Don’t forget to subscribe to the MED-EL Professionals Blog for weekly articles sent right to your inbox!
References:
- Behr, R., Colletti, V., Matthies, C., Morita, A., Nakatomi, H., Dominique, L., Darrouzet, V., Brill, S., Shehata-Dieler, W., Lorens, A., & Skarzynski, H. (2014). New outcomes with Auditory Brainstem Implants in NF2 Patients. Otol Neurotol.35(10): 1844–1851.
- Lundin, K., Stillesjö, F., Nyberg, G., & Rask-Andersen. (2016). Self-reported benefit, sound perception, and quality-of-life in patients with auditory brainstem implants (ABIs). H. Acta Otolaryngol. 136(1):62–67.
- Colletti, L., Shannon, R., & Colletti, V. (2014). The development of auditory perception in children following auditory brainstem implantation. Audiol Neurootol. 19(6): 386–394.
- Colletti, L., Shannon, R., Carner, M., Veronese, S., & Colletti, V. (2010) Complications in Auditory Brainstem Implant surgery in adults and children. Otol Neurotol. 31(4):558–564
- Behr, R., Müller, J., Shehata-Dieler, W., Schlake, H.P., Helms, J., Roosen, K., Klug, N., Hölper, B., Lorens, A. (2007) The high rate CIS Auditory Brainstem Implant for restoration of hearing in NF-2 patients. Skull Base. 17(2):91-107.
- Lundin, K., Stillesjö, F., Nyberg, G., & Rask-Andersen. (2016). Experiences from Auditory Brainstem Implantation (ABIs) in four paediatric patients. Cochlear Implants Int. 17(2):109–115.
- Shew, M., Bertsch, J., Camarata, P., & Staecker, H. (2017). Magnetic Resonance Imaging in a Neurofibromatosis Type 2 patient with a novel MRI-compatible Auditory Brainstem Implant. J Neurol Surg Rep. 78(1):e12-e14.
*Not all products, indications, and features shown are available in all areas. Please contact your local MED-EL representative for more information.
MED-EL
Was this article helpful?
Thanks for your feedback.
Sign up for newsletter below for more.
Thanks for your feedback.
Please leave your message below.
CTA Form Success Message
Send us a message
Field is required
John Doe
Field is required
name@mail.com
Field is required
What do you think?
The content on this website is for general informational purposes only and should not be taken as medical advice. Please contact your doctor or hearing specialist to learn what type of hearing solution is suitable for your specific needs. Not all products, features, or indications shown are approved in all countries.
Ayesha Amin
October 11, 2024
Assistance required
MED-EL
October 14, 2024
Hi Ayesha, thanks for your comment. If you require our local MED-EL team assistance, please contact us through this link: https://www.medel.com/contact-med-el. The team will reach out to you directly. Kind Regards, Giulia
Estefani
November 28, 2025
Necesito ayuda para mi bebé de 2 años 10 meses no es candidato para implante coclear debido a que su nervio auditivo no a desarrollado bien
MED-EL
December 01, 2025
Hi Esteafani, thank you for contacting us. We suggest contacting directly your local MED-EL team, so they can guide you through the options and assess the candidacy of your child personally. You can contact them via this form: https://www.medel.com/latam/contact-med-el. Kind Regards, Giulia
MED-EL




Conversation
2 Comments