Published Apr 12, 2024
The MED-EL Spotlight on BONEBRIDGE: Surgical Insights on Optimizing Clinical Practice & Outcomes

We recently met with Dr. David Kaylie of Duke University to hear his experience with incorporating BONEBRIDGE into his clinical practice over the last five years.

David Kaylie, MD
Duke University
BONEBRIDGE is MED-EL’s active transcutaneous bone conduction implant, which has been in use globally for more than a decade. The smart design of BONEBRIDGE is intended to leave surgeons and audiologists feeling confident in the device placement and in their patients’ long-term results.
Only BONEBRIDGE offers:
- A flexible implant for accommodating unique anatomies
- A low-profile design for reduced skin complications
- Two-point fixation for long-term stability
- Optimal magnetic retention for daily use
- An extensive list of convenience features with the SAMBA 2 audio processor
In Dr. Kaylie’s interview, we discuss which patients he has had the most success implanting BONEBRIDGE with and which BONEBRIDGE features have been the most beneficial for his practice. Read the interview highlights below.
Kensi Saia (KS): Which patient populations do you see who might be candidates for a bone conduction implant?
Dr. David Kaylie (DK): It’s really a couple populations. 1) People who have single-sided deafness (SSD) with no history of chronic ear disease but [only have] sudden sensorineural hearing loss or other reasons for SSD, and 2) people who have had chronic disease, a lot of extensive surgery, and big conductive hearing losses [are both really good candidates].
[These populations] may not be suitable for hearing aids, or [they] have difficulty with hearing aids, such as getting chronic infections with them. [This could be] someone who’s had a canal wall down [mastoidectomy procedure] and has very large [external auditory] meatus, and due to that, the hearing aid just doesn’t fit well. Those are also really good candidates.
KS: When did you start implanting BONEBRIDGE?
DK: I started doing BONEBRIDGE right when they became available. I implanted the first adult in the US, which was a week after the first US person, who was a child, was implanted.
The first adult was in 2019, and she’s still a very happy BONEBRIDGE recipient. She had lifelong SSD. When it was put on, she just burst into tears and just loved it.
KS: What initially sparked your interest in BONEBRIDGE?
DK: Several reasons. I was never really satisfied with percutaneous options because of all the skin issues and overgrowth. Patients just had a lot of trouble with it…A large percentage of them just had chronic infection, and it was just a constant trouble for them.
When fully subcutaneous options came about, I thought that was a really good option for my patients. There’s nothing through the skin and all of the energy is in the device in the bone.
KS: From a surgical perspective, is there specifically something about BONEBRIDGE that is suitable for your patients?
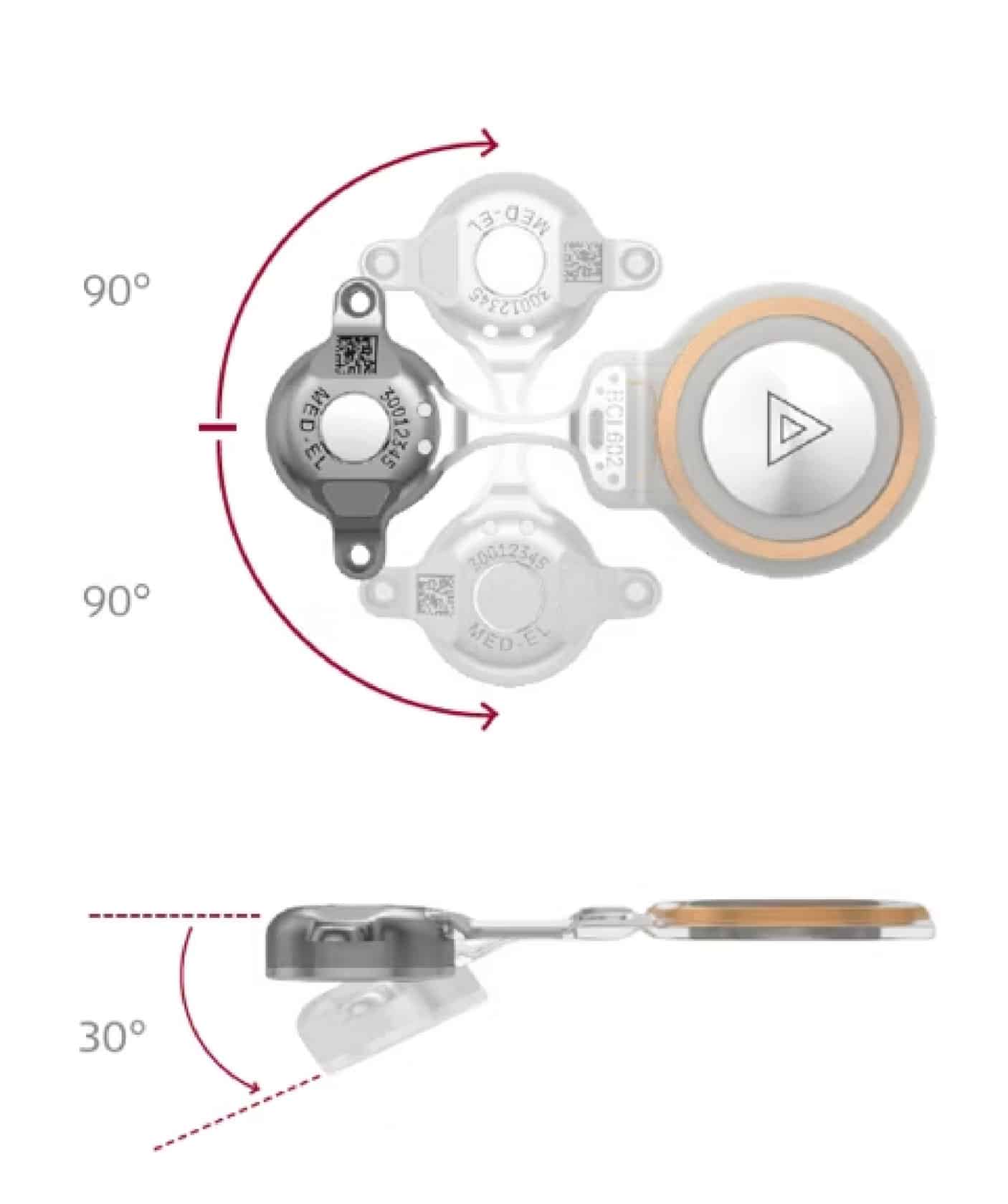
DK: I think the flexibility of it, meaning like it actually is flexible. You can bend it in multiple directions. It being truly flexible is the biggest strength for me, because I’m using it a lot in patients who’ve had prior mastoidectomies and prior canal wall down [mastoidectomies].
The BCI 602 can be bent so that the [SAMBA 2] device is at ear level, while the [BCI 602] implant is above the ear under the temporalis muscle. It’s very nice and flat, so there isn’t a bulge under the muscle.
[…] When you’re starting the surgery, you can take the template device and bend it how you want. You have a pretty good idea about the angle you are going to want it to be bent at. Then once you get in, sometimes the bone’s contour isn’t quite right. Sometimes you have to change the angle a little bit, but because it can bend so well, that’s never been a problem. You can always make it work.
KS: Do you feel like over time you’ve gotten more comfortable with placement of BONEBRIDGE over time?
DK: Absolutely. You know, very little can go wrong with it. The biggest thing is that you don’t want to make [the bed] too big because the flanges have to be anchored in the bone. If you’re doing it in the mastoid, it’s just easy—it is just a drill circle.
It’s incredibly stable, particularly because the flanges are recessed. You know that it is going in well because the screw ends up being flush in the flange’s recess. The two-point fixation is very, very secure and also ensures that it’s flush. I think that is a great feature of it.
Another is that they are not osseointegrated screws, they are just screws in the bone. They heal up quicker, and you don’t have to wait for osseointegration before you can [activate] it. The time after surgery to [activation] is very low.
The way we’re doing it, the receiver is in a sub-periosteum pocket, and so it is not creating a lot of swelling. The healing up time is quite quick. By three weeks, we’re activating [recipients].
KS: Are your practice’s audiologists heavily involved in the bone conduction device counseling process?
DK: Absolutely. We have a really comprehensive evaluation, where they receive information on BONEBRIDGE, CROS aids, and CochlearTM Osia®. There are certain cases where I feel like the BONEBRIDGE is really the only option for them. I will definitely let the audiologist know ahead of time that for medical reasons, BONEBRIDGE really is the best option for them. It’s a real team effort.
KS: Now that your patients have had the devices for a few years, have the outcomes been what you hoped they would be?
DK: [My patients] are very satisfied. I have had the fortune of having several patients switch from percutaneous to BONEBRIDGE or going from CROS aids to BONEBRIDGE. Uniformly, they say the sound quality is better. It’s an interesting A/B test for those people, and [those patients] are very satisfied that it is flush and that they don’t really feel it. There’s really not a cosmetic concern.
Getting people’s hearing back is just really exciting, particularly when a patient experienced trouble with another transcutaneous or percutaneous device, and now they’re not having that issue anymore. Or when a patient has chronic draining ear due to problems the hearing aids caused, and then they just don’t have that problem anymore [with BONEBRIDGE]. Not only do they hear better, but they don’t have all the infection nuisance, that is hugely impactful.
KS: Are patients returning to your clinic less often if they’re not having all of those additional complications?
DK: Absolutely, yeah. If you don’t have to manage chronic wound problems, then it’s better for everybody. Nobody wants to have to come to the doctor. Everybody has to take time out from their busy lives, and it can be really disruptive. So, if they can have that just be carefree and just come in for their routine checkups for their ear disease, that’s a big savings for them.
We’re always trying to maximize efficiency in clinic. And having fewer returns means that there’s more room for other [new] people. In a very busy practice, it just it just makes everything more efficient.
KS: What would you say to another surgeon who is considering implanting BONEBRIDGE?
DK: Well, I think the outcomes are phenomenal. There are some tricks to the surgery that I think are really important. Being able to implant it sub-temporalis gives you a much wider range of patients that you can do this for. I do think that is really important for someone who is starting out to make sure they understand that technique and really learn how to do that. [The technique] is very straightforward (i.e., drilling a circle). [BONEBRIDGE] really supports patients who have had a big mastoidectomy.
For someone who is starting out, I think if you can get the technique down, you can do anything.
Kensi Saia, AuD, PhD, CCC-A, is MED-EL US’s BONEBRIDGE product manager. Kensi earned her Doctor of Audiology and Doctor of Philosophy degrees from East Carolina University. Prior to joining MED-EL in 2018, she worked as a clinical audiologist with a focus on implantable devices.
David Kaylie, MD, MS, is a board-certified otologist and neurotologist. He is the professor of head and neck surgery and communication sciences, otology, and neurotology and skull base surgery at Duke University. Dr. Kaylie serves as the co-director of the Duke Skull Base Center, where he is developing novel ways to preserve and restore hearing after acoustic neuroma surgery using cochlear implants. He is also the Duke Vestibular Disorders Lab’s medical director, where they conduct testing and therapy for a wide range of balance problems and vertigo.
Want to hear more?
Watch the whole conversation between Dr. David Kaylie and MED-EL USA’s product manager of BONEBRIDGE.
Watch NowReferences
Was this article helpful?
Thanks for your feedback.
Sign up for newsletter below for more.
Thanks for your feedback.
Please leave your message below.
Thanks for your message. We will reply as soon as possible.
Send Us a Message
Field is required
John Doe
Field is required
name@mail.com
Field is required
What do you think?
© MED-EL Medical Electronics. All rights reserved. The content on this website is for general informational purposes only and should not be taken as medical advice. Contact your doctor or hearing specialist to learn what type of hearing solution suits your specific needs. Not all products, features, or indications are approved in all countries.


