Published Feb 08, 2024
The Latest Research on OTOPLAN and Anatomy-Based Fitting
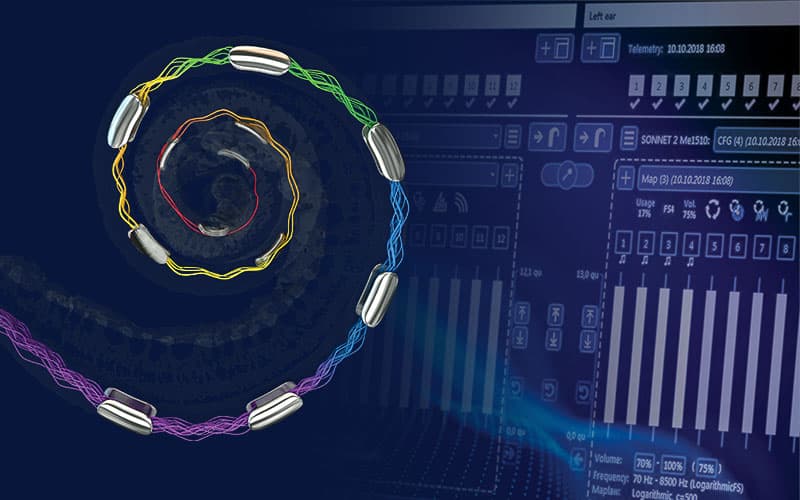
We have known for a century that the cochlear anatomy varies widely across individuals in both shape and size.Guild, S. R. (1921). A graphic reconstruction method for the study of the organ of Corti. Anat. Rec. 22, 140–157.[1]Hardy, M. (1938). The length of the organ of Corti in man. Am. J. Anat, 62, 291–311.[2]Koch, R. W., Ladak, H. M., Elfarnawany, M. & Agrawal, S. K. (2017). Measuring Cochlear Duct Length – a historical analysis of methods and results. J Otolaryngology - Head Neck Surg, 46, 19.[3] MED-EL recognized early that multiple electrode array length options are needed to address these differences. Over the last 30 years, we have expanded our electrode array portfolio to offer arrays from 20 to 31.5mm.
In 2022, MED-EL introduced another industry first with OTOPLAN* imaging software. OTOPLAN has revolutionized implant selection by helping surgeons select the optimal array for their patient based on their unique cochlear size.Yoshimura, H., Watanabe, K., Nishio, S., Takumi, Y., & Usami, S. (2023). Determining optimal cochlear implant electrode array with OTOPLAN. Acta Oto-Laryngologica, 143, 748 - 752.[4]
Why Does Choosing the Right Array Matter?
Modern cochlear implants divide incoming sounds into different frequency channels to represent pitch information. When a cochlear implant is activated, the acoustic information in each channel is converted into an electric signal that is delivered to an assigned electrode along the cochlear implant array.
Each electrode stimulates the adjacent auditory nerve fibers within the cochlea, collectively sending the signal through the auditory nerve to the brain. This allows the implant recipient to perceive sound.
Dividing sounds into multiple frequency channels allows cochlear implants to electrically stimulate specific cochlear locations to mimic how pitch information is delivered in natural hearing.
- High pitches are assigned to electrodes near the array’s base.
- Low pitches are assigned to electrodes near the array’s tip.
However, a patient’s cochlear size and the implanted array length greatly affect how well the pitch delivered through the implant matches the natural pitch of the nerve fibers at each electrode location.
One-Size-Fits-All Is Out, Individualization Is In
When a short electrode array is placed into an average or large cochlea, it creates a substantial discrepancy in pitch. This pitch mismatch may cause the recipient to perceive speech as unnaturally high-pitched and cartoon-like.Dorman, M. F., Natale, S. C., Baxter, L., Zeitler, D. M., Carlson, M. L., Lorens, A., Skarzynski, H., Peters, J. P. M., Torres, J. H., & Noble, J. H. (2020). Approximations to the Voice of a Cochlear Implant: Explorations With Single-Sided Deaf Listeners. Trends in hearing, 24, 2331216520920079. https://doi.org/10.1177/2331216520920079[5]
Historically, cochlear implant recipients have been implanted with the same array length regardless of their cochlear size. This means recipients with a small cochlear size may receive an array that provides optimal cochlear coverage, limiting pitch mismatch. Other recipients with larger cochlear size who are implanted with the very same array will have a result that is not ideal for their cochlear anatomy, creating a large pitch mismatch for them to overcome.
Recent evidence suggests that selecting an array that provides optimal cochlear coverage allows a cochlear implant recipient to quickly achieve the best benefit with their device and confirms that one size does not truly fit all cochleae.
- Patients who had closer to natural pitch delivered through their implant had significantly better understanding of words in quietCanfarotta, M. W., Dillon, M. T., Buss, E., Pillsbury, H. C., Brown, K. D., & O'Connell, B. P. (2020). Frequency-to-Place Mismatch: Characterizing Variability and the Influence on Speech Perception Outcomes in Cochlear Implant Recipients. Ear and hearing, 41(5), 1349–1361. https://doi.org/10.1097/AUD.0000000000000864[6] and sentences in noiseMertens, G., Van de Heyning, P.H., Vanderveken, O.M., Topsakal, V., & van Rompaey, V. (2021). The smaller the frequency-to-place mismatch the better the hearing outcomes in cochlear implant recipients? European Archives of Oto-Rhino-Laryngology, 279, 1875 - 1883.[7] in the first six months after implantation compared to patients with larger levels of pitch mismatch.
- Patients who received free-fitting, atraumatic, straight arrays providing 79-82% cochlear coverage had significantly better word recognition scores than patients with less cochlear coverage.Weller, T., Timm, M. E., Lenarz, T., & Büchner, A. (2023). Cochlear coverage with lateral wall cochlear implant electrode arrays affects post-operative speech recognition. PloS one, 18(7), e0287450. https://doi.org/10.1371/journal.pone.0287450[8]
Studies like these confirm that individualized array selection for the right fit is a crucial component of optimizing patient outcomes, and the array length choice should not be overlooked by a one-size-fits-all approach.
For implanted patients, OTOPLAN allows for additional opportunities to reduce pitch mismatch. With postoperative OTOPLAN analysis, clinicians have access to:
- The specific location of each implanted electrode contact in their patient’s cochlea.
- The frequencies associated with each contact’s location using state-of-the-art techniques developed through SYNCHROTRON imaging.Li, H., Helpard, L., Ekeroot, J., Rohani, S.A., Zhu, N., Rask-Andersen, H., Ladak, H. M., Agrawal, S. (2021). Three-dimensional tonotopic mapping of the human cochlea based on synchrotron radiation phase-contrast imaging. Scientific Reports, 11(1), 4437.[9]
This information can be easily imported into MED-EL’s MAESTRO programming software† to allow for Anatomy-Based Fitting.
Fine-Tune Pitch with Anatomy-Based Fitting
Anatomy-Based Fitting is MED-EL’s frequency fitting option that offers clinicians an objective tool for aligning frequency information (delivered through the patient’s individualized program on their audio processor) with their unique anatomical frequency information obtained from OTOPLAN.
Ongoing US and European research studies are actively investigating how using anatomy-based information for frequency programming impacts patient outcomes.
University of North Carolina
Dr. Margaret Dillon is conducting a clinical trial in newly implanted cochlear implant recipients comparing performance outcomes between patients programmed with anatomy-based techniques and patients programmed with the traditional “one-size-fits-all” frequency setting.
“The motivation to conduct research on the effectiveness of eliminating frequency-to-place mismatches came from the results of earlier studies…[where] we found that patients implanted with long electrode arrays had better speech recognition…than patients implanted with a shorter electrode array.
In a follow-up study, we found that these differences persisted with long-term cochlear implant use. The hypothesis was that the patients with the long electrode array had better outcomes because the default filter frequencies were closer to the natural cochlear place frequency…
We thought using imaging to view the placement of each electrode and then individualizing the filter information to match the cochlear place frequency would support patients in hearing their best.”
Margaret Dillon, AuD, PhD
The University of North Carolina at Chapel Hill
Initial results published on a group of participants using electric-acoustic stimulation suggested that using anatomy-based programming to reduce pitch mismatch shortly after implantation achieves similar outcomes to optimal array placement.Dillon, M. T., Canfarotta, M. W., Buss, E., Rooth, M. A., Richter, M. E., Overton, A. B., Roth, N. E., Dillon, S. M., Raymond, J. H., Young, A., Pearson, A. C., Davis, A. G., Dedmon, M. M., Brown, K. D., & O'Connell, B. P. (2023). Influence of Electric Frequency-to-Place Mismatches on the Early Speech Recognition Outcomes for Electric-Acoustic Stimulation Users. American journal of audiology, 32(1), 251–260. https://doi.org/10.1044/2022_AJA-21-00254[10]
University of California at San Francisco
Dr. Charles Limb and Dr. Melanie Gilbert at the University of California at San Francisco are also conducting a clinical trial where they are using anatomical information from flat-panel CT scans to program new cochlear implant recipients.
Participants using anatomy-based programming are tested over the first year of device use for speech understanding using experimental tests designed by the investigators to explore music perception and sound quality. Participants were also tested using the traditional frequency setting after one year.
“Music sound quality is very important for many CI recipients, many of whom are musicians or audiophiles, and we aspire to use customized, anatomy-based fitting to decrease the mismatch. Music relies on accurate pitch information, and we are showing that decreasing the mismatch, at least in new CI recipients, may improve music perception as well as speech understanding.”
Melanie Gilbert, AuD
University of California, San Francisco
Preliminary results suggest that using anatomy-based programming techniques provided participants with improved speech understanding in quiet. Most participants reported an increased pleasantness of music.Gilbert, M. L., Deroche, M. L. D., Jiradejvong, P. & Limb, C. J. (2023). Longitudinal Crossover Study of Music and Speech Perception of CI Cohort Utilizing Flat Panel CT-Based Mapping at Initial Activation. In Dallas, TX.[11]
Germany
The University of Würzburg reported that experienced bilateral recipients may see improved speech understanding and sound localization outcomes when anatomical information is used to align pitch across ears.Kurz, A., Herrmann, D., Hagen, R., & Rak, K. (2023). Using Anatomy-Based Fitting to Reduce Frequency-to-Place Mismatch in Experienced Bilateral Cochlear Implant Users: A Promising Concept. Journal of personalized medicine, 13(7), 1109. https://doi.org/10.3390/jpm13071109[12]
France
Researchers found that the combined use of MED-EL’s fine structure coding and frequency adjustments using anatomical information from OTOPLAN yielded significantly better detection of speech sounds in noise compared to traditional programming approaches.Creff, G., Lambert, C., Coudert, P., Pean, V., Laurent, S., & Godey, B. (2024). Comparison of Tonotopic and Default Frequency Fitting for Speech Understanding in Noise in New Cochlear Implantees: A Prospective, Randomized, Double-Blind, Cross-Over Study. Ear and hearing, 45(1), 35–52. https://doi.org/10.1097/AUD.0000000000001423[13]
Looking Towards the Future
Anatomy-Based Fitting offers a new tool for clinicians to optimize pitch programming for both new and existing patients and adds a dimension to cochlear implant fitting that has never been available to audiologists until now. Ongoing research will continue to refine our understanding of how to better represent frequency information in the natural cochlea.Helpard, L., Li, H., Rohani, S. A., Zhu, N., Rask-Andersen, H., Agrawal, S., & Ladak, H. M. (2021). An Approach for Individualized Cochlear Frequency Mapping Determined From 3D Synchrotron Radiation Phase-Contrast Imaging. IEEE transactions on bio-medical engineering, 68(12), 3602–3611. https://doi.org/10.1109/TBME.2021.3080116[14]
Dr. Sumit Agrawal and Dr. Hanif Ladak at Western University are conducting studies using leading-edge imaging techniques through SYNCHROTRON scanning to create high resolution 3D images of the cochlea and surrounding structures.
They aim to improve upon the knowledge obtained from past histological studies—which relied on looking at the tiny cochlear structures under a microscope—and were the foundational studies which established understanding of cochlear anatomy and how pitch is naturally coded.Greenwood D. D. (1990). A cochlear frequency-position function for several species--29 years later. The Journal of the Acoustical Society of America, 87(6), 2592–2605. https://doi.org/10.1121/1.399052[15]Stakhovskaya, O., Sridhar, D., Bonham, B. H., & Leake, P. A. (2007). Frequency map for the human cochlear spiral ganglion: implications for cochlear implants. Journal of the Association for Research in Otolaryngology : JARO, 8(2), 220–233. https://doi.org/10.1007/s10162-007-0076-9[16] This work offers a promising future for further patient programming optimization, and MED-EL is excited to partner with these investigators to continue to push the boundary of patient outcomes.Leighton, E. (2023). Donation of $8.5 million strengthens hearing science and innovation, Western News.[17]
* OTOPLAN is a product of CASCINATION AG.
† MAESTRO Cochlear Implant Fitting Software version 9.0.5 or later and a SONNET 2, SONNET 2 EAS, or RONDO 3 is required.
References
-
[1]
Guild, S. R. (1921). A graphic reconstruction method for the study of the organ of Corti. Anat. Rec. 22, 140–157.
-
[2]
Hardy, M. (1938). The length of the organ of Corti in man. Am. J. Anat, 62, 291–311.
-
[3]
Koch, R. W., Ladak, H. M., Elfarnawany, M. & Agrawal, S. K. (2017). Measuring Cochlear Duct Length – a historical analysis of methods and results. J Otolaryngology – Head Neck Surg, 46, 19.
-
[4]
Yoshimura, H., Watanabe, K., Nishio, S., Takumi, Y., & Usami, S. (2023). Determining optimal cochlear implant electrode array with OTOPLAN. Acta Oto-Laryngologica, 143, 748 – 752.
-
[5]
Dorman, M. F., Natale, S. C., Baxter, L., Zeitler, D. M., Carlson, M. L., Lorens, A., Skarzynski, H., Peters, J. P. M., Torres, J. H., & Noble, J. H. (2020). Approximations to the Voice of a Cochlear Implant: Explorations With Single-Sided Deaf Listeners. Trends in hearing, 24, 2331216520920079. https://doi.org/10.1177/2331216520920079
-
[6]
Canfarotta, M. W., Dillon, M. T., Buss, E., Pillsbury, H. C., Brown, K. D., & O’Connell, B. P. (2020). Frequency-to-Place Mismatch: Characterizing Variability and the Influence on Speech Perception Outcomes in Cochlear Implant Recipients. Ear and hearing, 41(5), 1349–1361. https://doi.org/10.1097/AUD.0000000000000864
-
[7]
Mertens, G., Van de Heyning, P.H., Vanderveken, O.M., Topsakal, V., & van Rompaey, V. (2021). The smaller the frequency-to-place mismatch the better the hearing outcomes in cochlear implant recipients? European Archives of Oto-Rhino-Laryngology, 279, 1875 – 1883.
-
[8]
Weller, T., Timm, M. E., Lenarz, T., & Büchner, A. (2023). Cochlear coverage with lateral wall cochlear implant electrode arrays affects post-operative speech recognition. PloS one, 18(7), e0287450. https://doi.org/10.1371/journal.pone.0287450
-
[9]
Li, H., Helpard, L., Ekeroot, J., Rohani, S.A., Zhu, N., Rask-Andersen, H., Ladak, H. M., Agrawal, S. (2021). Three-dimensional tonotopic mapping of the human cochlea based on synchrotron radiation phase-contrast imaging. Scientific Reports, 11(1), 4437.
-
[10]
Dillon, M. T., Canfarotta, M. W., Buss, E., Rooth, M. A., Richter, M. E., Overton, A. B., Roth, N. E., Dillon, S. M., Raymond, J. H., Young, A., Pearson, A. C., Davis, A. G., Dedmon, M. M., Brown, K. D., & O’Connell, B. P. (2023). Influence of Electric Frequency-to-Place Mismatches on the Early Speech Recognition Outcomes for Electric-Acoustic Stimulation Users. American journal of audiology, 32(1), 251–260. https://doi.org/10.1044/2022_AJA-21-00254
-
[11]
Gilbert, M. L., Deroche, M. L. D., Jiradejvong, P. & Limb, C. J. (2023). Longitudinal Crossover Study of Music and Speech Perception of CI Cohort Utilizing Flat Panel CT-Based Mapping at Initial Activation. In Dallas, TX.
-
[12]
Kurz, A., Herrmann, D., Hagen, R., & Rak, K. (2023). Using Anatomy-Based Fitting to Reduce Frequency-to-Place Mismatch in Experienced Bilateral Cochlear Implant Users: A Promising Concept. Journal of personalized medicine, 13(7), 1109. https://doi.org/10.3390/jpm13071109
-
[13]
Creff, G., Lambert, C., Coudert, P., Pean, V., Laurent, S., & Godey, B. (2024). Comparison of Tonotopic and Default Frequency Fitting for Speech Understanding in Noise in New Cochlear Implantees: A Prospective, Randomized, Double-Blind, Cross-Over Study. Ear and hearing, 45(1), 35–52. https://doi.org/10.1097/AUD.0000000000001423
-
[14]
Helpard, L., Li, H., Rohani, S. A., Zhu, N., Rask-Andersen, H., Agrawal, S., & Ladak, H. M. (2021). An Approach for Individualized Cochlear Frequency Mapping Determined From 3D Synchrotron Radiation Phase-Contrast Imaging. IEEE transactions on bio-medical engineering, 68(12), 3602–3611. https://doi.org/10.1109/TBME.2021.3080116
-
[15]
Greenwood D. D. (1990). A cochlear frequency-position function for several species–29 years later. The Journal of the Acoustical Society of America, 87(6), 2592–2605. https://doi.org/10.1121/1.399052
-
[16]
Stakhovskaya, O., Sridhar, D., Bonham, B. H., & Leake, P. A. (2007). Frequency map for the human cochlear spiral ganglion: implications for cochlear implants. Journal of the Association for Research in Otolaryngology : JARO, 8(2), 220–233. https://doi.org/10.1007/s10162-007-0076-9
-
[17]
Leighton, E. (2023). Donation of $8.5 million strengthens hearing science and innovation, Western News.
References
Was this article helpful?
Thanks for your feedback.
Sign up for newsletter below for more.
Thanks for your feedback.
Please leave your message below.
Thanks for your message. We will reply as soon as possible.
Send Us a Message
Field is required
John Doe
Field is required
name@mail.com
Field is required
What do you think?
© MED-EL Medical Electronics. All rights reserved. The content on this website is for general informational purposes only and should not be taken as medical advice. Contact your doctor or hearing specialist to learn what type of hearing solution suits your specific needs. Not all products, features, or indications are approved in all countries.



