Published Nov 12, 2024
FDA Expands MED-EL Criteria for Adults with Residual Hearing

On October 3, 2024, the Food and Drug Administration (FDA) approved new indications for MED-EL cochlear implants, providing access to more adults with residual hearing. This approval bridges the gap in candidacy between traditional and electric-acoustic stimulation (EAS) indications Pillsbury, H. C. 3rd, Dillon, M.T., Buchman, C. A., Staecker, H., Prentiss, S. M., Ruckenstein, M. J., Bigelow, D. C., Telischi, F. F., Martinez, D. M., Runge, C.L., Friedland, D. R., Blevins, N. H., Larky, J. B., Alexiades, G., Kaylie, D. M., Roland, P. S., Miyamoto, R. T., Backous, D. D., Warren, F. M.,… Adunka, O. F. (2018). Multicenter US Clinical Trial With an Electric-Acoustic Stimulation (EAS) System in Adults: Final Outcomes. Otology & Neurotology, 39(3), 299-305. https://doi.org/10.1097/MAO.0000000000001691.[1] and confirms hearing preservation rates with FLEX electrode arrays.
Less than 10% of eligible candidates receive a cochlear implant Carlson, M.L. (2020). Cochlear Implantation in Adults. New England Journal of Medicine, 382(16), 1531-1542. https://doi.org/10.1056/NEJMra1904407.[2],Nassiri, A.M., Sorkin, D.L., Carlson, M.L. (2022). Current Estimates of Cochlear Implant Utilization in the United States. Otology & Neurotology, 43(5), e558-e562. https://doi.org/10.1097/MAO.0000000000003513.[3]. Broader cochlear implant indications and public awareness are important to improve utilization rates in the United States (US). Many candidates with residual hearing delay treatment for fear of losing their remaining hearing. Conventional thinking has been that all remaining hearing will be lost when a cochlear implant is placed. However, MED-EL’s unique FLEX electrode arrays are designed to minimize the risk of damage to the cochlea during insertion, and the data supporting this FDA approval show that FLEX electrodes indeed are able to preserve residual hearing for many recipients.
Hearing Preservation with FLEX Electrode Arrays
MED-EL studied hearing preservation rates with FLEX electrode arrays in a multicenter clinical trial and a registry study. The multicenter study shows that 53.6% of clinical trial participants with MED-EL’s longer FLEX electrodes (28-31.5 mm) still had aidable hearing in the implant ear 6 months after cochlear implant fitting. Aidable hearing is defined by capabilities of MED-EL audio processors with an acoustic unit, which can provide gain for unaided thresholds up to 80 dB HL at 125-1000 Hz.
The European registry collects real-word data without strict inclusion/exclusion criteria and does not control for surgical techniques used to preserve residual hearing. Long-term hearing preservation for registry data was defined using the Vienna Consensus and the American Academy of Otolaryngology (AAO) reporting standards Adunka, O. F., Gantz, B.J., Dunn, C., Gurgel, R. K., Buchman, C. A. (2018). Minimum Reporting Standards for Adult Cochlear Implantation. Otolaryngology–Head and Neck Surgery, 159(2), 215-219. https://doi.org/10.1177/0194599818764329.[4]. The Vienna Consensus was developed in 2023 by an advisory board of 24 surgeons from different countries and defines hearing preservation based on the shift in low-frequency pure-tone average (LFPTA) at 250, 500, and 1000 Hz.
According to the Vienna Consensus, a shift of 15 dB HL or less is considered complete hearing preservation, and a shift of 16-30 dB HL is partial hearing preservation. In the real-world registry population, 57.4% of MED-EL FLEX electrode recipients still had complete or partial hearing preservation as long as 1-2 years after surgery. Based on the AAO reporting standards, many MED-EL users also still have functionally-relevant residual hearing 1-2 years after surgery with a FLEX electrode, defined as a LFPTA better than 80 dB HL at 125, 250, and 500 Hz.
Multicenter Clinical Trial Results
Five academic medical centers implanted 44 adults in the US and Canada at a mean age of 68 years. Study participants had a bilateral moderate to profound sensorineural hearing loss (SNHL), defined by a LFPTA greater than 40 dB HL at 250, 500, and 1000 Hz, and high-frequency thresholds not better than 65 dB HL at 3000-8000 Hz. Figure 1 shows mean speech perception scores across study intervals.
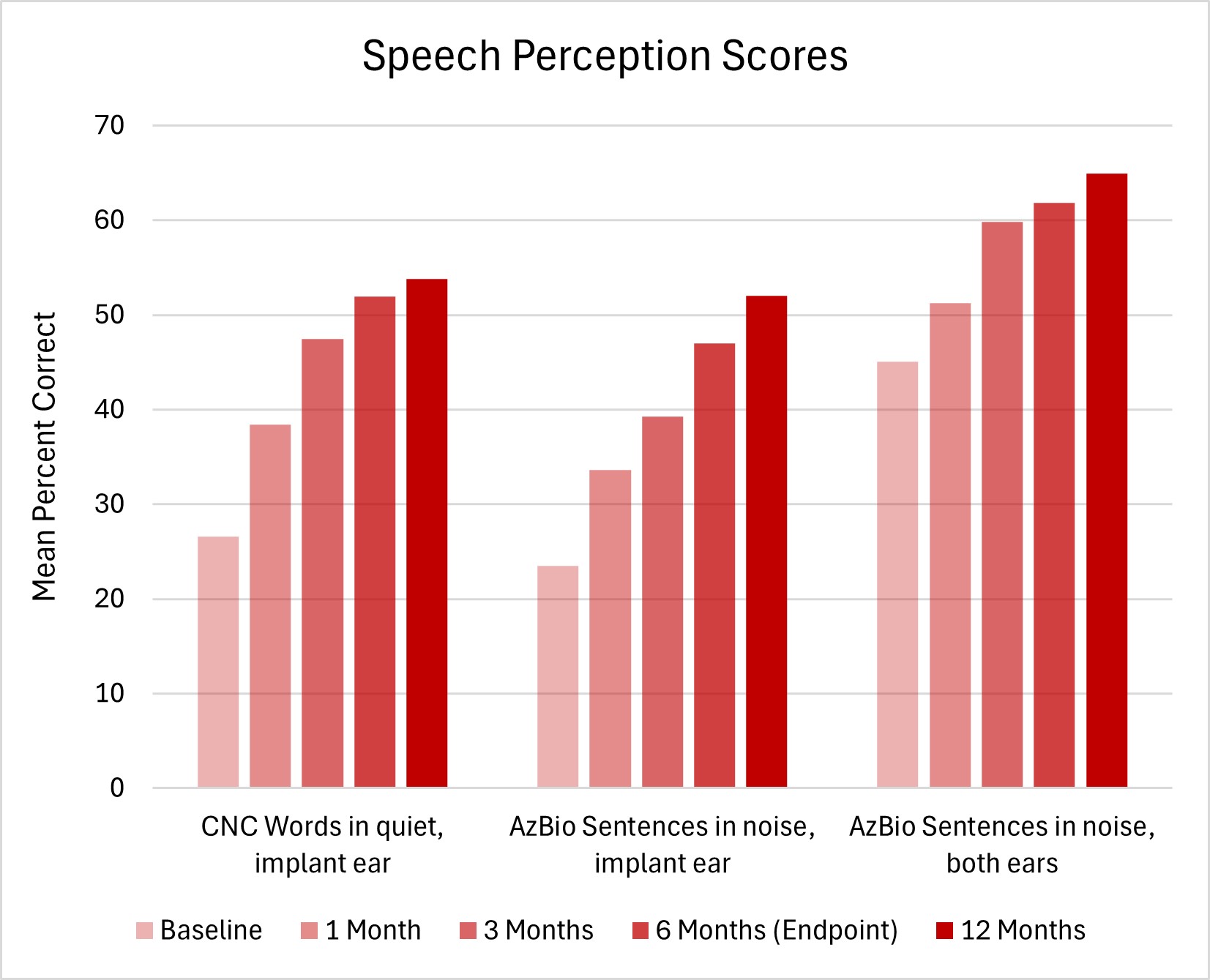
As a group, mean scores improved significantly on Consonant-Nucleus-Consonant (CNC) Words in quiet and AzBio Sentences in the implant ear alone 6 months after fitting compared to baseline scores with hearing aids. Nearly all adults (90.7%) implanted with a FLEX28 or FLEXSOFT electrode array score better on CNC Words in quiet and/or AzBio Sentences in noise by 6 months after fitting. Even when hearing cannot be preserved, most MED-EL cochlear implant users understand words in quiet and/or sentences in noise better by 6 months after fitting in the implant ear alone compared to baseline scores with hearing aids.
FDA Approves Expanded Indications
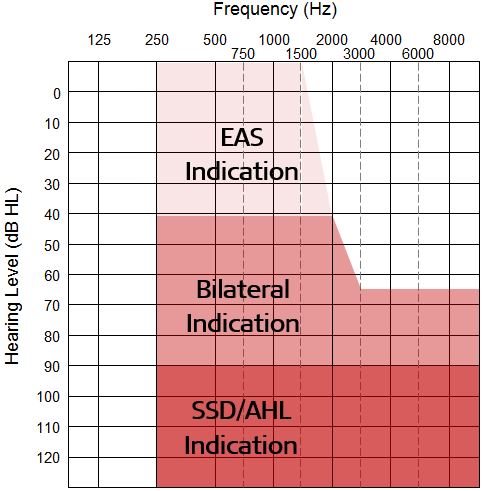
Multicenter clinical trial results show that MED-EL cochlear implants are safe and effective for adults with bilateral moderate to profound SNHL who obtain limited benefit from appropriately-fit hearing aids. This includes people 18 years of age and older with a LFPTA greater than 40 dB HL at 250 Hz, 500 Hz, and 1000 Hz and high-frequency thresholds not better than 65 dB HL at 3000-8000 Hz. Limited benefit from hearing aids means CNC word scores of 50% or less in the ear to be implanted and 60% or less in the non-implant ear.
This approval is the first of its kind, reflecting the hearing preservation results of the unique FLEX electrode design. Only MED-EL offers long electrode arrays which are available in multiple lengths to fit each individual’s cochlea.
Implications and Next Steps
Candidates who meet MED-EL’s FDA-approved indications should not delay treatment due to fear of losing their residual hearing. While loss of residual hearing remains a risk with any cochlear implant surgery, it is no longer a given that the surgery will destroy all remaining hearing with MED-EL FLEX electrodes. MED-EL’s unique FLEX electrode design shows some degree of hearing preservation 1-2 years after surgery with all electrode array lengths (24-31.5 mm).
MED-EL is the first cochlear implant approved by the FDA for adults with any degree of low-frequency residual hearing, bridging the gap between traditional and EAS indications. Figure 2 below shows MED-EL’s audiologic candidacy criteria for people 18 years of age and older.
Public awareness on advances in cochlear implant indications, benefits, and hearing preservation rates can improve cochlear implant use and outcomes in the US. MED-EL’s FLEX electrode arrays are uniquely designed to preserve residual hearing, and MED-EL cochlear implant users show significant improvement across electrode array lengths and varying degrees of hearing preservation. Candidates and clinicians should not delay seeking treatment or referring for a cochlear implant evaluation.
References
-
[1]
Pillsbury, H. C. 3rd, Dillon, M.T., Buchman, C. A., Staecker, H., Prentiss, S. M., Ruckenstein, M. J., Bigelow, D. C., Telischi, F. F., Martinez, D. M., Runge, C.L., Friedland, D. R., Blevins, N. H., Larky, J. B., Alexiades, G., Kaylie, D. M., Roland, P. S., Miyamoto, R. T., Backous, D. D., Warren, F. M.,… Adunka, O. F. (2018). Multicenter US Clinical Trial With an Electric-Acoustic Stimulation (EAS) System in Adults: Final Outcomes. Otology & Neurotology, 39(3), 299-305. https://doi.org/10.1097/MAO.0000000000001691.
-
[2]
Carlson, M.L. (2020). Cochlear Implantation in Adults. New England Journal of Medicine, 382(16), 1531-1542. https://doi.org/10.1056/NEJMra1904407.
-
[3]
Nassiri, A.M., Sorkin, D.L., Carlson, M.L. (2022). Current Estimates of Cochlear Implant Utilization in the United States. Otology & Neurotology, 43(5), e558-e562. https://doi.org/10.1097/MAO.0000000000003513.
-
[4]
Adunka, O. F., Gantz, B.J., Dunn, C., Gurgel, R. K., Buchman, C. A. (2018). Minimum Reporting Standards for Adult Cochlear Implantation. Otolaryngology–Head and Neck Surgery, 159(2), 215-219. https://doi.org/10.1177/0194599818764329.
References
Was this article helpful?
Thanks for your feedback.
Sign up for newsletter below for more.
Thanks for your feedback.
Please leave your message below.
Thanks for your message. We will reply as soon as possible.
Send Us a Message
Field is required
John Doe
Field is required
name@mail.com
Field is required
What do you think?
© MED-EL Medical Electronics. All rights reserved. The content on this website is for general informational purposes only and should not be taken as medical advice. Contact your doctor or hearing specialist to learn what type of hearing solution suits your specific needs. Not all products, features, or indications are approved in all countries.



