Tip Fold-Over & Perimodiolar Cochlear Implant Electrodes: Research Update
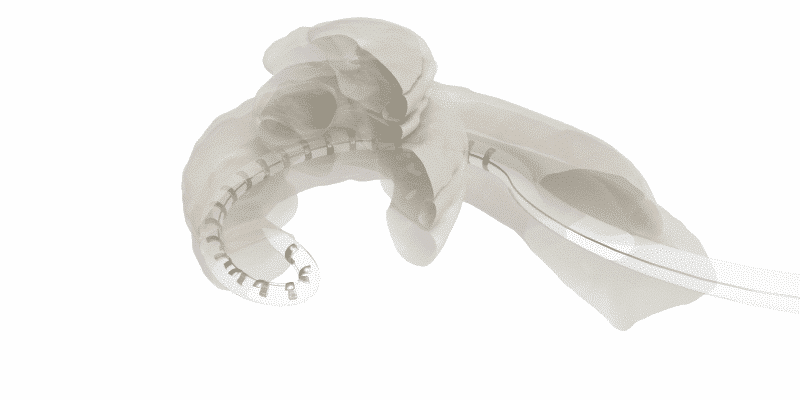
Above all else, the goal of cochlear implants is to create a useful interface between the electrode array and the neural structures of cochlea—to create a bridge between technology and nature.
If the electrode array cannot effectively stimulate the cochlea, there is a bottleneck in the communication pathway. Any benefit of front-end processing or assistive listening accessories would be, in effect, hobbled by a comprised connection between the implant array and the auditory nerve.
Considering the entire purpose of a cochlear implant relies on the quality of this interface between electrode array and the cochlea, there is an astounding contrast in the outcomes between different electrode designs.
Recently, multiple centers have reported an emerging complication—tip fold-over.1,2,3
Basically, in tip fold-over or tip rollover, the electrode array folds over upon itself. This seriously compromises the interface between the electrode contacts and the cochlea, and often limits coverage to less than ¾ of a single turn in the cochlea.
Of course, the overlapping and unpredictable location of the folded-over electrode contacts would likely cause fitting difficulties for the audiologist and would reduce the overall benefit for the patient.1,2,3,4,5,6
Tip fold-over of an electrode array may lead to:
- Vertigo
- Overlapping electrodes
- Reduced insertion depth
- Revision surgery or electrode reinsertion
- Deviation into the scala vestibuli
- Damage to the delicate structures of the cochlea
- Deactivation of multiple electrodes
- Limited mapping potential
- Distorted sound quality
- Poor hearing performance
- Reduced overall benefit from cochlear implant
When a perimodiolar array tip folds over, this can severely limit stimulation range and can damage cochlear structures.1,2,3,4
Over the past months, there has been an increasing number of research articles highlighting this complication. Recent multi-center research has further confirmed that this is a surprisingly common adverse event with certain electrode arrays.1,2,3,4
Thankfully, this extensive research has also shown that MED-EL has the lowest reported incidence of tip fold-over.1,7
Let’s look at why tip fold-over is not an issue with MED-EL, how you can detect tip fold-over with other arrays, and the implications for cochlear implant surgeons, audiologists, and patients.
Avoiding Tip Fold-Over
How can you avoid electrode tip fold-over? As this complication has been frequently reported even by experienced surgeons, the best course of action may be to avoid using electrode designs that are prone to folding over.
MED-EL electrode arrays are the most flexible and atraumatic arrays available, so it’s logical to investigate if tip fold-over is possible with MED-El electrode arrays.8,9,10,11,12
It’s important to understand that MED-EL’s unique flexible lateral wall arrays are free-fitting, and the rounded tip of the array gently adapts to the anatomy of each individual cochlea. This enables reliably atraumatic scala tympani placement and the lowest reported rate of tip fold-over.8,9,10,12,15-24
Most importantly, we can look at the evidence. Gabreilpillai et al. (2018) recently published an extensive retrospective analysis of 1,320 cochlear implant recipients (1,722 ears) with post-operative imaging. The University Hospital Frankfurt is a large academic center with experienced surgeons.
 778 flexible MED-EL lateral wall arrays were implanted—post-operative imaging confirmed that there were no cases of tip fold-over. In contrast, more than 10% of Slim Modiolar CI532 arrays folded over. Contour AdvanceTM and Slim Straight arrays also folded over in multiple cases, though less frequently.1
778 flexible MED-EL lateral wall arrays were implanted—post-operative imaging confirmed that there were no cases of tip fold-over. In contrast, more than 10% of Slim Modiolar CI532 arrays folded over. Contour AdvanceTM and Slim Straight arrays also folded over in multiple cases, though less frequently.1
McJunkin et al. and Lang et al. also recently reported 6–8% tip fold-over rates with the Slim Modiolar CI532 electrode. In contrast, a multi-center review of imaging from 216 MED-EL implants confirmed there were 0 reported cases of tip fold-over. With such large datasets available, it’s clear that electrode choice makes a distinct difference in outcomes.1,2,3,4,5
Detecting Tip Fold-Over
One of the main challenges of tip fold-over is that it may not be noticeable to the implanting surgeon. McJunkin et al. (2018) reported that due to limited tactical feedback, this adverse event is often not perceivable to the surgeon.2
Intraoperative Detection
Furthermore, current intraoperative neural response telemetry or impedance measurements are not a reliable means of identifying tip fold-overs. This means that additional intraoperative imaging is necessary to confirm placement when using an array that carries a relatively high risk of tip fold-over.1,2,3
Gabrielpillai et al. (2018) reported that x-ray alone was not sufficient to confirm tip fold-over in every case, so additional CT imaging was needed. Lang et al. (2019) states that intraoperative DVT (digital volume tomography) imaging is obligatory to avoid revision surgery when using slim modiolar arrays.1,3
Of course, many centers may not have CT or DVT immediately and conveniently available for every CI procedure. In any case, needing to transfer the patient to radiology intraoperatively is not considered an ideal workflow.
Post-operative detection
Furthermore, many instances of tip fold-over are not detected until post-operatively. Without surgical revision, overlapping electrode contacts need to be deactivated. This limits stimulation range and significantly impacts hearing outcomes and patient benefit.1,2,3,4 On the other hand, returning to the OR for revision surgery in the days or weeks following implantation would also be a significant burden for both patients and clinicians.
Reliable Performance
The purpose of a cochlear implant electrode array is to provide a precisely positioned interface to the intricate neural structures of the cochlea. That’s why MED-EL arrays are specifically engineered to deliver reliable scala tympani placement and complete cochlear coverage for the closest possible match to natural hearing for your patients.
And with MED-EL’s full-length flexible arrays, your patients can benefit from:10,13,14
- Complete cochlear coverage
- More natural place-pitch match
- Much more natural sound quality
- Significant improvement in hearing in short time
- Significantly better hearing in noise & quiet
*Not all products, indications, and features shown are available in all areas. Please contact your local MED-EL representative for more information.
References
- Gabrielpillai, J., Burck, I., Baumann, U., Stoever, T., Helbig, S. (2018) Incidence for Tip Foldover During Cochlear Implantation. Otology Neurotology, 39(9): 1115–1121.
- McJunkin, J.,L., Durakovic, N., Herzog, J., & Buchman, C.,A. (2018) Early Outcomes With a Slim, Modiolar Cochlear Implant Electrode Array. Otol Neurotol. 39(1):e28-e33.
- Lang, C.P., Salcher, R., Timm, M., Teschner, M,. Lenarz, T. (2018) Tip Fold-over with the Slim Modiolar electrode (CI 532), a retrospective case series. Laryngo-Rhine-Otol. 97(S 02): S211
- Zuniga, M.G., Rivas, A., Hedley-Williams, A., Gifford, R.H., Dwyer, R., Dawant, B.M., Sunderhaus, L.W., Hovis, K.L., Wanna, G.B., Noble, J.H., & Labadie, R.F. (2016) Tip fold-over in cochlear implantation: Case series. Otol Neurotol. 38(2):199–206.
- Cosetti, M.K., Troob, S.H., Latzman, J.M., Shapiro, W.H., Roland, J.T. Jr., & Waltzman, S.B. (2012) An evidence-based algorithm for intraoperative monitoring during cochlear implantation. Otol Neurotol. 33: 169–176.
- Grolman W, Maat A, Verdam F, et al. (2009) Spread of excitation measurements for the detection of electrode array fold-overs: A prospective study comparing 3-dimensional rotational x-ray and intraoperative spread of excitation measurements. Otol Neurotol 30:27–33.
- MED-EL data on file. Based on retrospective analysis of post-operative imaging of 216 MED-EL implants from multiple centers.
- O’Connell, B.P., Hunter, J.B., & Wanna, G.B., (2016). The importance of electrode location in cochlear implantation. Laryngoscope Investigative Otolaryngology, 1: 169–174.
- Wanna, G.B., Noble, J.H., Carlson, M.L., Gifford, R.H., Dietrich, M.S., Haynes, D.S., Dawant, B.M., & Labadie, R.F. (2014). Impact of electrode design and surgical approach on scalar location and cochlear implant outcomes. Laryngoscope. 124(6):1–7.
- O’Connell, B.P., Cakir, A., Hunter, J.B., Francis, D.O., Noble, J.H., Labadie, R.F., Zuniga, G., Dawant, B.M., Rivas, A., & Wanna, G.B. (2016). Electrode location and angular insertion depth are predictors of audiologic outcomes in cochlear implantation. Otol Neurotol. 37(8):1016–1023.
- Boyer, E., Karkas, A., Attye, A., Lefournier, V., Escude, B., & Schmerber, S. (2015) Scalar localization by cone-beam computed tomography of cochlear implant carriers: A comparative study between straight and periomodiolar precurved electrode arrays. Otol Neurotol. 36(3):422–429.
- Nordfalk, K., Rasmussen, K., Hopp, E., Bunne, M., Silvola, J.T., & Jablonski, G.E., (2016). Insertion Depth in Cochlear Implantation and Outcome in Residual Hearing and Vestibular Function. Ear Hear. 37(2):e129–137.
- Buchman, C.A., Dillon, M.T., King, E.R., Adunka, M.C., Adunka, O.F., & Pillsbury, H.C. (2014). Influence of cochlear implant insertion depth on performance: a prospective randomized trial. Otol Neurotol., 35(10), 1773–1779.
- Helbig, S., Helbig, M., Leinung, M., Stöver, T., Baumann, U., & Rader, T. (2015). Hearing preservation and improved speech perception with a flexible 28-mm electrode. Otol Neurotol. 2015 Jan;36(1):34-42.
- Aschendorff, A., Briggs, R., Brademann, G., Helbig, S., Hornung, J., Lenarz, T., Marx, M., Ramos, A., Stoever, T., Escude, B., & James, C.J. (2017). Clinical investigation of the Nucleus Slim Modiolar Electrode. Audiol Neurootol. 22(3). 169–179
- Friedmann, D.R., Kamen, E., Choudhury, B., & Roland, J.T. (2019). Surgical experience and early outcomes with a slim perimodiolar electrode. Otol Neurotol. 40(3).
- Gomez Serrano, M., Patel, S., Harris, R., & Selvadurai, D. (2019).
Initial surgical and clinical experience with the Nucleus CI532 slim modiolar electrode in the UK. Cochlear Implants Int. 20(4).207–216. - Jia, H., Torres, R., Nguyen, Y., De Seta, D., Ferrary, E., Wu, H., Sterkers, O., Bernardschi, D., & Mosnier, I. (2018). Intraoperative conebeam CT for assessment of intracochlear positioning of electrode arrays in adult recipients of cochlear implants. AJNR Am J Neuroradiol. 39(4). 768–774.
- Lohmann, A.R., Carlson, M.L., & Sladen, D.P. (2018). Intraoperative cochlear implant device testing utilizing an automated remote system: A prospective pilot study. Otol Neurotol. 39(3). 313–317.
- Sipari, S., Iso-Mustajarvi, M., Lopponen, H., & Dietz, A. (2018). The insertion results of a Mid-Scala electrode assessed by MRI and CBCT image fusion. Otol Neurotol. 39(10).
- Stefanescu, E.H. & Motoi, S. (2018). Selection of the appropriate cochlear electrode array using a specifically developed research software application. J Laryngol Otol. 132(6). 544–549.
- Timm, M.E., Majdani, O., Weller, T., Windeler, M., Lenarz, T., Buechner, A. & Salcher, R.B. (2018). Patient specific selection of lateral wall cochlear implant electrodes based on anatomical indication ranges. PLoS ONE 13(10).
- Mittmann, P., Lauer, G., Ernst, A., Mutze, S., Hassepass, F., Arndt, S., Arweiler-Harbeck, D., & Christov, F. (2020). Electrophysiological detection of electrode fold over in perimodiolar cochlear implant electrode arrays: a multi center study case series. Eur Arch Otorhinolaryngol. 277(1). 31–35.
CTA Form Success Message
Send us a message
Field is required
John Doe
Field is required
name@mail.com
Field is required
What do you think?
The content on this website is for general informational purposes only and should not be taken as medical advice. Please contact your doctor or hearing specialist to learn what type of hearing solution is suitable for your specific needs. Not all products, features, or indications shown are approved in all countries.