MRI Scans with MED-EL Cochlear Implants
With an ever-increasing use of MRI, there’s essentially a 100% likelihood a person will need at least one, if not multiple, MRI scans in their lifetime. In fact, in just the United States, more than 100,000 MRI scans are performed every day.
That means every cochlear implant will very likely end up inside the powerful magnetic field of a high-field MRI scanner. It might be a routine knee scan a few months after implantation, or an emergency spine MRI following a car accident years from now, but that day is coming. That’s what makes MRI safety* such an essential consideration for every single cochlear implant recipient.
Unfortunately, many cochlear implants carry a significant risk of complications during MRI, including pain, discomfort, and magnet dislocation. Why is there such a risk? The answer is simple—it comes down to the design of the implant’s internal magnet. 1,2,3,4,5,6,7,8,9
Every cochlear implant has a magnet inside—but not all cochlear implant magnets are designed the same. In 2014, MED-EL revolutionized CI magnet design with our SYNCHRONY cochlear implant. SYNCHRONY introduced the world’s first rotatable, self-aligning diametric CI magnet that enabled 3.0 Tesla MRI without magnet removal.
However, it’s also important to remember that cochlear implants are made to last for many years, so many recipients are happily using implants that are already 5, 10, or even 20+ years old. This is crucial in terms of MRI safety, because this means the vast majority of cochlear implants currently being used by CI recipients from all companies are likely implants with earlier-generation magnet technology.
When we talk about MRI safety, we can’t just look at what’s possible today in terms of MRI safety with the most advanced cochlear implants. Instead, we have to look at what is realistically encountered every day by radiologists, clinicians, and recipients.
Great Protection. Guaranteed.
For more than 25 years, we have engineered our cochlear implants to offer reliable MRI safety. Our cochlear implants enable immediate and reliable access to MRI scans for both recipients and clinicians. Whether your patient has an implant from our SYNCHRONY Series, CONCERTO Series, SONATA Series, PULSAR, or even all the way back to our COMBI 40/COMBI 40+ implants, they’ll have access to MRI without surgery (unless required for diagnostic reasons) or risk of magnet dislocation—all our CIs from the past 25 years, with any electrode array, following straightforward conditions. With MED-EL cochlear implants, your patients can have MRI where they need it, when they need it.
In fact, MED-EL is the first and only cochlear implant company to offer an MRI guarantee for cochlear implants that covers all of our multichannel cochlear implants going back to 1994.*** This life-long and worldwide guarantee covers all MRI scans that follow the simple, straightforward conditions laid out in our medical procedure manual. You can find the full MRI conditions for all MED-EL implants at medel.com/isi.
MED-EL’s multichannel cochlear and brainstem implants are covered in addition to the mandatory local statutory guarantee by a MED-EL life-long and worldwide guarantee against:
- Implant damage,
- Implant magnet dislocation,
- Implant magnet demagnetization,
- implant damage due to magnet removal as performed according to Instructions for Use/Manual for Medical Procedures for MED-EL CI/ABI Systems
as an unlikely consequence of an MRI scan.
Why is this so important for you and your patients, as well as radiologists and other clinicians?4,6,9
- Safe, comfortable access to MRI scans
- Immediate access to MRI
- Saves time & resources for health care providers
- Peace of mind & reliability
Let’s look at what makes MED-EL implants the right choice for safe, reliable access to MRI scans.4,5,6,9
And if you’re looking for our detailed MRI instructions, make sure to read through the full MRI conditions for all MED-EL implants at medel.com/isi before performing the MRI.
Powerful Magnetic Fields
First, let’s look at the design of MRI machines. Every magnetic resonance imaging scanner has very powerful magnets. These are static magnets, which means they’re usually always at full magnetic force, even if the scanner isn’t running.
Those static magnets are incredibly strong—strong enough to lift a car. Right now, 1.5 Tesla is the most common field strength, but high-resolution 3.0 Tesla scanners are quickly gaining popularity.
Now, let’s look at cochlear implants. As mentioned, every cochlear implant has an internal magnet to hold the audio processor communication coil in place. Newer generations of cochlear implants use rotatable, self-aligning magnets that greatly reduce magnet issues during MRI. MED-EL introduced this innovation in 2014, with other companies following with similar options starting in 2018–2019.
There is an ever-growing number of CI recipients with the latest implants, but the majority of existing CI recipients are using earlier generation implants, where a simple axial magnet is used, with a magnetization perpendicular to the skin. An axial magnet is like refrigerator magnet; north is one side and south is on the other side.
Generally, the “head” end of a scanner is the main magnet’s north or south pole, with the opposite pole centered at the entrance of the scanner. An implant lies flat against the skull, so that axial implant magnet is oriented perpendicular to the main static magnetic field of the scanner.
As a patient enters the scanner, the implant magnet will attempt to align to this powerful field, so this 90° offset can cause significant torque force that pulls on the magnet. This powerful torque force is the main challenge for MRI scans with a cochlear implant.
Magnet Design & Retention
There are two main implant design factors that affect what happens to the implant magnet during an MRI scan: what type of magnet is used and how that magnet is held in the implant.
- What type of magnet the implant uses:
- Axial magnets are simple magnets with a basic north-south polarization, similar to a standard refrigerator magnet. The magnetization is perpendicular to the skin. These simple magnets hold an audio processor work well for holding audio processor in place during everyday use, but can lead to issues of magnetic torque or “pulling” during MRI if they are not secure.
- Rotatable diametric magnets are a newer design that has a “sideways” magnetization (i.e. the magnetization is parallel to the skin). Since the magnet can freely rotate it can better align to the magnetic field. This greatly reduces any magnetic torque, even in more powerful 3.0 Tesla scanners.9
- How the magnet is secured in the implant:
- Soft silicone pocket designs use a thin lip of silicone or silastic material to hold the magnet in place during everyday use. However, this is not sufficient retention for an axial magnet during an MRI, so the design is intended to allow the magnet to be surgically removed for MRI. Rotatable magnet designs reduce torque force, so they can be left in place.7
- Securely embedded magnets are firmly fixed inside the implant itself, distributing torque force over a wide footprint and preventing any risk of the magnet dislocating during an MRI.
- Securely removable magnets combine security of a locking magnet housing with the advantage of being able to optionally remove the magnet for reduced image artifact on brain scans near the implant.10,11
Soft Silicone Pocket: Magnet Dislocation
An overwhelming majority of reported CI-related MRI issues involve an “axial magnet in a soft silicone pocket” design.3,4,5,6,7,8,9,12 Thankfully, the newer rotatable, self-aligning magnet generation should help greatly reduce these magnetic torque issues for recipients with the newest magnet technology. However, as we have seen, the vast majority of implant users do not have the newest generation of implants. Even today, these earlier implants with axial magnets are still commonly implanted in many countries.
For earlier axial magnet designs, most other manufacturers have used a “soft-silicone pocket” design, which has been in use since 1997. This concept uses a thin lip of silicone around the rim of an exposed magnet for retention. This design is intended to allow the magnet to be surgically removed before an MRI scan.1,2
However, there was a trend to perform a 1.5 T MRI with magnet in place, even with devices which didn’t have regulatory approval for this. The idea behind was to avoid “unnecessary magnet surgeries” if implant magnet dislocation could be prevented, because temporary magnet explantation & reimplantation is not without risk.5,12
Unfortunately, the soft silicone pocket design also often allows the axial magnet to be unintentionally removed during a scan by the magnetic force of the MRI scanner. During a scan, an axial magnet will attempt to twist out of the pocket to align with the magnetic field of the scanner. The soft silicone pocket only partially covers the magnet and provides only minimal resistance to magnet dislocation.3,4,5,6,7
Magnet dislocation is an alarmingly frequent issue that continues to be encountered by radiologists and clinicians. To reduce the rate of dislocation, other cochlear implant manufacturers require that the magnet must either be surgically removed or a rigid splint system and tight head bandage must be applied over the implant before any 1.5 Tesla scan.1,2
The rigid splint puts pressure against the skin above the magnet to resist the forces of dislocation. However, this doesn’t reduce the magnetic torque. The magnet can still attempt to align to the magnetic field. This can create highly concentrated pressure against the skin pinched between the rigid splint and magnet.
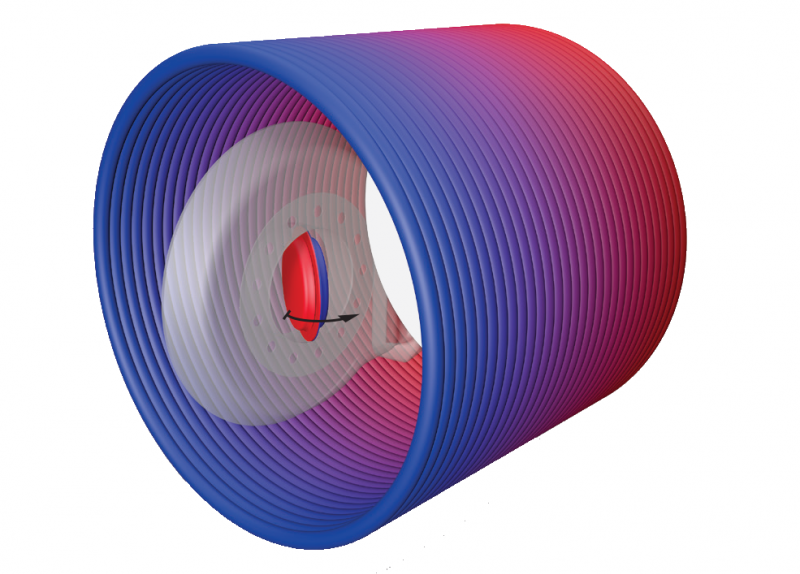
If an axial magnet is not securely imbedded, it can be dislocated by the powerful magnetic field of the MRI scanner. Magnet dislocation can cause the magnet to tilt up to 90° on edge to align with the north-south field of the scanner.
Even without full magnet dislocation, the severe pain and discomfort of this pressure can make it impossible to complete an MRI scan. And with a rigid splint in place, these magnets can still dislocate during a 1.5 Tesla MRI, which can cause extreme pain and may require unplanned surgical intervention to relocate or replace the magnet. Recently, a major manufacturer revoked MRI approval in the United States using a splint kit for their soft silicone pocket implants with axial magnet in situ, which means surgical removal would be required for any MRI scan. 3,4,5,6,8
With this inherent risk of magnet displacement or dislocation, complications are common with axial magnets in soft-silicone pocket designs. These complications are well documented, with complication rates during 1.5 Tesla scans ranging even as high as 30–55%. This may deter clinicians from performing the MRI, which could delay effective diagnosis or treatment. In case of an emergency, such as a car accident, immediate access to MRI can be seriously impeded.3,4,5,6,8
Soft Silicone Pocket: Implant Damage
Furthermore, during intentional or unintentional magnet removal, the thin silicone pocket could possibly tear, damaging the implant and necessitating complete removal of the damaged implant and reimplantation with an entirely new cochlear implant.
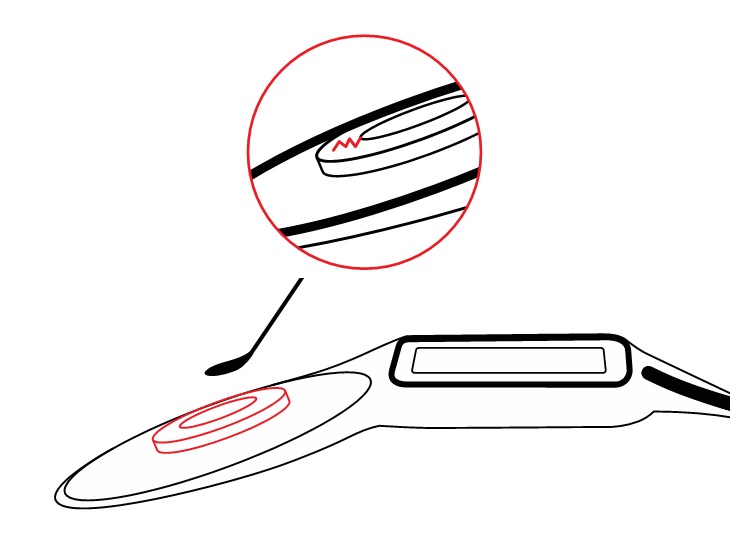 Every magnet removal/replacement cycle can require several surgical interactions with this soft silicone pocket: removal of the magnet, insertion of a non-magnetic spacer, MRI scan, removal of the non-magnetic spacer, and insertion of a new magnet.
Every magnet removal/replacement cycle can require several surgical interactions with this soft silicone pocket: removal of the magnet, insertion of a non-magnetic spacer, MRI scan, removal of the non-magnetic spacer, and insertion of a new magnet.
Even the newest generations of implants with a soft silicone pocket or cassette inside a silastic housing are at risk of damage to the implant during magnet removal and replacement.
Requiring an entirely new cochlear implant replacement surgery would be a serious burden on any patient and healthcare providers. Walker et al. (2018) describes this issue with soft silastic pocket designs as “a potentially debilitating and costly complication of pursuing magnet removal.”3,8 And if a magnet can’t be safely removed without risk of damaging the implant, then is that really any benefit?
SAR Limits: 3.0 T ≠ 3.0 T
Generally with implanted medical devices, magnet dislocation is not always the greatest concern. With implants like pacemakers, deep brain stimulators or spinal cord stimulators, one of the main risks is tissue damage caused by heating of the long electrode leads. Thankfully, cochlear implants use much shorter electrode leads, so this risk is greatly reduced.
However, the are many different scan conditions that need to be followed beyond magnet procedure, and these can vary even within a company’s implant series. One important parameter is a control of the amount of radio energy absorbed by the patient and their implant, known as the Specific Absorption Rate (SAR).
For example, one of the newest implants available widely promotes 3.0 Tesla MRI access, but 3.0 Tesla scans with this implant need to be limited to a whole body averaged specific absorption rate (SAR) of <0.4 W/kg. Even at 1.5 Tesla, many implants have relatively restrictive SAR limits.
Why is this so important? If SAR values need to be very restricted, this can limit scanning options for the radiologist. This can also affect the quality of the imaging and may require longer scanning sessions. Tighter SAR limits also may reduce the safety margin, making it more difficult to proceed with the scan in an everyday setting.
As you can see, for implants from other companies, a new generation of magnet design hasn’t necessarily solved many of the practical challenges faced by radiologists, clinicians, and recipients in the field. And for many CI recipients with earlier implants from other providers, there are significant risks that can hinder access to MRI scans.
Secure Magnet Retention
Remarkably, these complications are not an issue with MED-EL hearing implants, including our cochlear implants from more than 25 years ago. How is this possible?3,4,5,6,9
Beginning in 1994, MED-EL was the first company to offer cochlear implants that were specially designed to offer safe, comfortable access to MRI. For more than 25 years, our earlier generations of MED-EL cochlear implants have featured a fixed axial magnet securely embedded within the implant, making magnet dislocation practically impossible.
In fact, with more than 100,000 implants, there has never been a single reported case of magnet dislocation with a MED-EL CI. This secure design has a very practical advantage: With any MED-EL multichannel cochlear implant, you can perform a 1.5 Tesla scan without any risk of magnet dislocation.
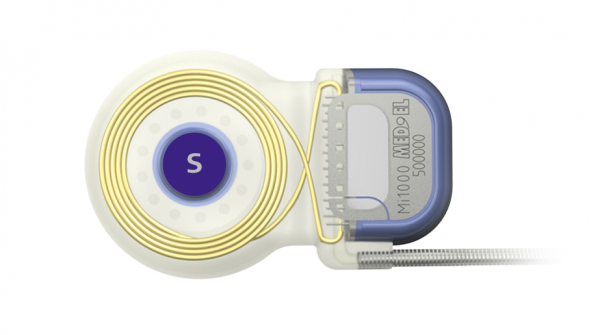
A MED-EL cochlear implant with an axial cochlear implant magnet securely imbedded in silicone with force-distributing stiffening ring.
Inside of an MRI scanner, this secure axial magnet design evenly distributes any magnetic torque across the full surface area of the implant coil, which helps avoid any painful focused pressure. For these implants, a simple head bandage should be used to help minimize any movement.
Of course, a reasonable SAR limit is also important for useful imaging. Thankfully, all of our multichannel cochlear implants since 1994 are approved for 1.5 Tesla scans in Normal Operating Mode, which means 3.2 W/kg head SAR and 2.0 W/kg whole body averaged SAR, when following the conditions in the instructions for use.
- CONCERTO, MED-EL CONCERT, SONATA, PULSAR, C40+, C40
- 1.5 Tesla MRI in Normal Operation Mode following conditions in instructions for use
- No risk of magnet dislocation
- Simple head bandage to support implant
- Immediate return to hearing after scan
BONEBRIDGE & VIBRANT SOUNDBRIDGE
We don’t just provide an MRI guarantee with our cochlear implants—every recipient deserves MRI safety, so it’s a priority for our entire portfolio.
Our BONEBRIDGE BCI 601 & BCI 602 Active Bone Conduction Implants and VIBRANT SOUNDBRIDGE VORP 503 Middle Ear Implant use a secure, force-balanced magnet that also enables comfortable 1.5 Tesla MRIs. Even better, these implants are designed to minimize any torque-related movement in an MRI, so no head bandage is needed. These implants are also fully covered by our MRI guarantee.***
- BONEBRIDGE BCI 601, BONEBRIDGE BCI 602, & SOUNDBRIDGE VORP 503
- 1.5 Tesla MRI in Normal Operating Mode following conditions in instructions for use
- No risk of magnet dislocation
- No head bandage needed
- Immediate return to hearing after scan
- Outstanding MRI safety
*Please note the earlier VIBRANT SOUNDBRIDGE VORP 502 Active Middle Ear Implant is not approved for MRI scans, so it is labelled MR Unsafe and should not be scanned.
3.0 Tesla MRI Safety
And then we have the name that changed the game in cochlear implants and MRI: SYNCHRONY.
Launched in 2014, our SYNCHRONY cochlear implant is designed to safely provide immediate access to 1.5 & 3.0 Tesla MRI at any time—without magnet removal and without any head bandage. With a revolutionary diametric magnet design, SYNCHRONY eliminates the negative effects of magnetic torque on the implant magnet, even at 3.0 Tesla.
The rotatable diametric magnet self-aligns to the magnetic field of the scanner, so there’s no uncomfortable force on the implant. This means recipients with SYNCHRONY can have a 3.0 Tesla scan without any need for a split kit or a supportive head wrap.9
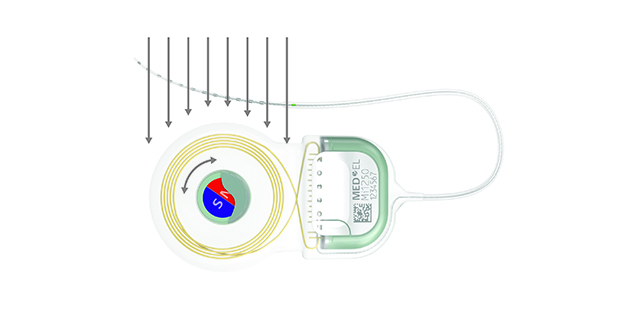 SYNCHRONY’s diametric magnet can rotate and self-align inside of a secure titanium housing.
SYNCHRONY’s diametric magnet can rotate and self-align inside of a secure titanium housing.
Furthermore, the exceptionally secure magnet is practically impossible to accidentally dislodge. If you need clear imaging of the brain directly adjacent to the implant, the SYNCHRONY magnet can optionally be safely and easily removed. 10,12
Thanks to the unique conical locking mechanism, the SYNCHRONY magnet is secured by the full thickness of the implant coil. This robust design enables repeated magnet removal and replacement cycles without damaging the implant.
The great majority of MRI scans don’t require clearer ipsilateral brain imaging, so magnet removal is very rare with SYNCHRONY, but we have a long history of going above and beyond when it comes to delivering the best in MRI safety for recipients and practical benefits for clinicians.
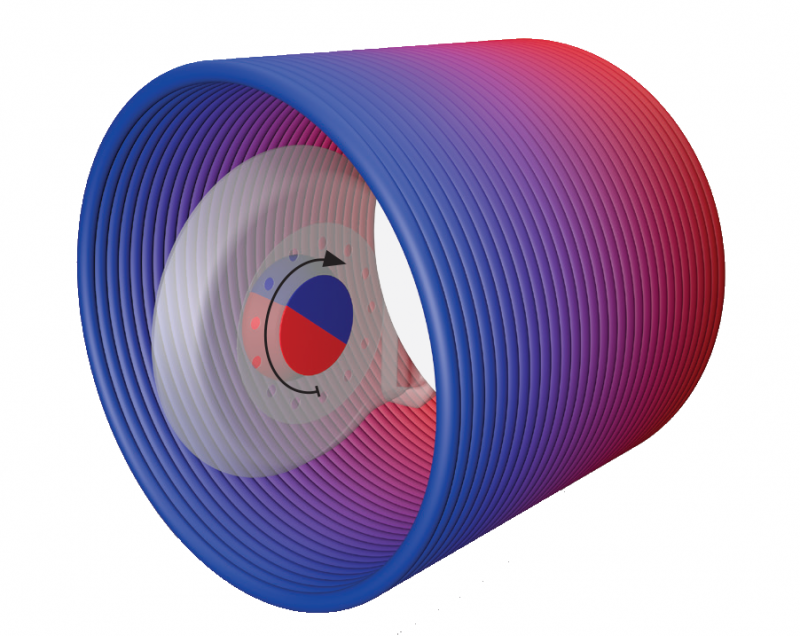 Inside of an MRI scanner, SYNCHRONY’s magnet rotates to align with the main static magnetic field, so there’s practically no magnetic torque force.
Inside of an MRI scanner, SYNCHRONY’s magnet rotates to align with the main static magnetic field, so there’s practically no magnetic torque force.
For practical access to a wide variety of scan sequences, SYNCHRONY offers reasonable SAR conditions. For all 1.5 Tesla scans, Normal Operating Mode is approved. For 3.0 Tesla scans, Normal Operation Mode is approved for body scans for regions >35 cm from the top of the head, with separate limits for head and neck scans. Make sure to follow all conditions covered in the medical procedures manual at www.medel.com/isi.
For 1.5 or 3.0 Tesla scans with SYNCHRONY, there’s no need for magnet removal, rigid splints, or even head bandaging. Your patient simply removes their audio processor for the scan, while the radiology team follows a few simple precautions. Your patient can return to using their audio processor right away after leaving the scanning room.9
- SYNCHRONY 2 Cochlear Implant, SYNCHRONY Cochlear Implant
- 1.5 Tesla & 3.0 Tesla MRI without magnet removal
- No risk of magnet dislocation
- No head bandage needed
- Immediate return to hearing after scan
Peace of Mind
Looking forward, your patients will all need safe, reliable access to MRI scans. And they rely on you to help them make the best-informed decision they can. With MED-EL, you can help ensure they’ll have safety, comfort, and peace of mind for years to come.
This simplicity offers more than convenience; it provides much greater safety and security for your patients and fellow clinicians, while ensuring immediate access to essential diagnostic imaging.
With MED-EL, your patients can have MRI where they need it, when they need it.
Subscribe & Share
Looking for more information on MRI with MED-EL hearing implants? Check out our step-by-step MRI guides for cochlear implants and for SOUNDBRIDGE & BONEBRIDGE.
Want first-hand perspective on MRI with SYNCHRONY? Check out this fascinating MRI case study: “Our patient and device have undergone seven MRIs of the head, C spine, and T spine without any issues or demagnetization to the device while still providing quality images.”
Have a radiology question about MED-EL hearing implants? Contact your local MED-EL office or let us know with our simple contact form.
Want more in-depth articles on hearing implant technology? Subscribe now to get our latest articles right to your inbox!
*MED-EL cochlear implants since 1994 are MR conditional. Recipients with a MED-EL cochlear implant may be safely MRI scanned following the conditions detailed in the Medical Procedures Manual.
**Not all products, indications, and features shown are available in all areas. Please contact your local MED-EL representative for more information.
*** The terms and conditions of the MRI guarantee can be found here.
References
- Cochlear MRI guidelines for professionals. https://www.cochlear.com/us/en/professionals/resources-and-training/mri-guidelines
- MRI Safety Information with the Advanced Bionics HiRes™ Ultra Cochlear Implant. https://advancedbionics.com/us/en/home/professionals/mri-safety.html
- Hassepass, F., Stabenau, V., Arndt, S., Beck, R., Bulla, S., Grauvogel, T., & Aschendorff, A. (2014) Magnet dislocation: an increasing and serious complication following MRI in patients with cochlear implants. Rofo. 186 (7) 680–685
- Kim, B.G., Kim, J.W., Park, J.J., Kim, S.H., Kim, H.N., & Choi, J.Y. (2015). Adverse events and discomfort during magnetic resonance imaging in cochlear implant recipients. JAMA Otolaryngol Head Neck Surg. 141(1), 45–52.
- Carlson, M.L., Neff, B.A., Link, M.J., Lane, J.I., Watson, R.E., McGee, K.P., Bernstein, M.A., & Driscoll, C.L. (2015) Magnetic resonance imaging with cochlear implant magnet in place: safety and imaging quality. Otol Neurotol. 36(6):965–971.
- Young, N.M., Rojas, C., Deng, J., Burrowes, D., & Ryan, M. (2016) Magnetic resonance imaging of cochlear implant recipients. Otol Neurotol. 37(6):665–671.
- Gubbels, S., & McMenomey, S. (2006) Safety study of the Cochlear Nucleus 24 device with internal magnet in the 1.5 Tesla magnetic resonance imaging scanner. Laryngoscope. 116(6):865–871.
- Walker, B., Norton, S., Phillips, G., Christianson, E., Horn, D., & Ou, H. (2018) Comparison of MRI in pediatric cochlear implant recipients with and without retained magnet. Int. Journal of Pediatric Otorhinolaryngology. Epub ahead of print. https://doi.org/10.1016/j.ijporl.2018.03.013
- Todt, I., Tittel, A., Ernst, A., Mittmann, P., Mutze, S. (2017) Pain free 3 T MRI scans in cochlear implantees. Otol Neurotol. 38(10) e401–e404.
- Wagner, F., Wimmer, W., Leidolt, L., Vischer, M., Weder, S., Wiest, R., Mantokoudis, G., & Caversaccio, M. (2015) Significant artifact reduction at 1.5T and 3T MRI by the use of a cochlear implant with removable magnet: an experimental human cadaver study. PLoS One. 10(7): e0132483.
- Helbig, S., Stoever, T., Burck, I., Kramer, S. (2017) Cranial MRI in a young child with cochlear implants after bilateral magnet removal. Int J Pediatr Otorhinolaryngol. 103:1-4.
- Pross, S.E., Ward, B.K., Sharon, J.D., Weinreich, H.M., Aygun, N., Francis, H.W. (2018). A prospective study of pain from magnetic resonance imaging with cochlear implant magnets in situ. Otol Neurotol. 39(2):e80-e86.
- Franceschi, A.M, Wiggins, G.C., Mogilner, A.Y., Shepherd, T., Chung, S., & Lui, Y.W. (2016). Optimized, Minimal Specific Absorption Rate MRI for High-Resolution Imaging in Patients with Implanted Deep Brain Stimulation Electrodes. AJNR Am J Neuroradiol. 37(11):1996-2000
CTA Form Success Message
Send us a message
Field is required
John Doe
Field is required
name@mail.com
Field is required
What do you think?
The content on this website is for general informational purposes only and should not be taken as medical advice. Please contact your doctor or hearing specialist to learn what type of hearing solution is suitable for your specific needs. Not all products, features, or indications shown are approved in all countries.



